How Many Valence Electrons In Argon
News Leon
Mar 23, 2025 · 6 min read
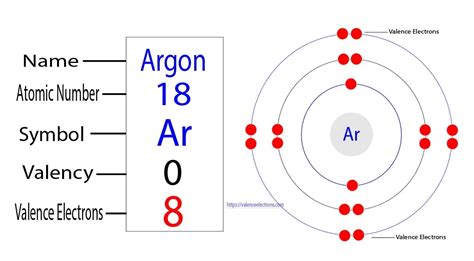
Table of Contents
- How Many Valence Electrons In Argon
- Table of Contents
- How Many Valence Electrons Does Argon Have? A Deep Dive into Electron Configuration and Chemical Properties
- Understanding Valence Electrons: The Key to Chemical Reactivity
- The Octet Rule: A Foundation of Chemical Bonding
- Argon's Electron Configuration: A Stable Octet
- Argon's Valence Electrons: A Count of Eight
- Why Eight Valence Electrons Means Inertness
- Argon's Properties and Applications: A Consequence of its Valence Electrons
- 1. Inert Atmosphere for Welding and Metallurgy:
- 2. Protective Atmosphere for Lighting and Electronics:
- 3. Cryogenics:
- 4. Scientific Instruments and Analysis:
- 5. Medical Applications:
- Argon's Position in the Periodic Table and its Relationship to Valence Electrons
- Comparing Argon to Other Noble Gases:
- Beyond the Basics: Understanding Ionization Energy and Electronegativity
- Ionization Energy: The Energy to Remove an Electron
- Electronegativity: The Attraction for Electrons
- Conclusion: Argon's Inertness – A Testament to its Eight Valence Electrons
- Latest Posts
- Latest Posts
- Related Post
How Many Valence Electrons Does Argon Have? A Deep Dive into Electron Configuration and Chemical Properties
Argon, a noble gas with the symbol Ar and atomic number 18, is a fascinating element with unique properties stemming directly from its electron configuration. A key aspect of understanding argon's behavior lies in determining its number of valence electrons. This article delves deep into the concept of valence electrons, explores argon's electron configuration, and explains why its valence electron count dictates its chemical inertness and other important properties. We'll also examine how this understanding contributes to various applications of argon.
Understanding Valence Electrons: The Key to Chemical Reactivity
Valence electrons are the electrons located in the outermost shell or energy level of an atom. These electrons are the most loosely bound to the nucleus and, therefore, participate directly in chemical bonding. The number of valence electrons determines an element's reactivity and the types of chemical bonds it can form. Elements with a full outermost shell (typically eight electrons, following the octet rule) are generally unreactive, while those with fewer than eight valence electrons tend to be more reactive, seeking to gain, lose, or share electrons to achieve a stable octet.
The Octet Rule: A Foundation of Chemical Bonding
The octet rule states that atoms tend to gain, lose, or share electrons in order to have eight electrons in their valence shell. This configuration is considered exceptionally stable due to the complete filling of the s and p orbitals in the outermost shell. Exceptions to the octet rule exist, particularly with elements in the third period and beyond, but it remains a fundamental principle for understanding chemical bonding.
Argon's Electron Configuration: A Stable Octet
Argon's atomic number is 18, meaning it has 18 protons and 18 electrons in a neutral atom. Its electron configuration is 1s²2s²2p⁶3s²3p⁶. Let's break this down:
- 1s²: Two electrons in the first energy level, filling the 1s orbital.
- 2s²: Two electrons in the second energy level, filling the 2s orbital.
- 2p⁶: Six electrons in the second energy level, filling the three 2p orbitals.
- 3s²: Two electrons in the third energy level, filling the 3s orbital.
- 3p⁶: Six electrons in the third energy level, filling the three 3p orbitals.
Notice that the third energy level (n=3), the outermost shell, is completely filled with eight electrons (2 in the 3s and 6 in the 3p orbitals). This completely filled outermost shell is the key to understanding argon's chemical behavior.
Argon's Valence Electrons: A Count of Eight
Based on argon's electron configuration, it's clear that argon has eight valence electrons. These eight electrons occupy the 3s and 3p orbitals, forming a complete and stable octet. This stable electron configuration is the primary reason why argon is a noble gas and exhibits extremely low reactivity.
Why Eight Valence Electrons Means Inertness
Having a complete octet of valence electrons makes argon exceptionally stable. It has no tendency to gain, lose, or share electrons to achieve a more stable configuration because it already possesses the most stable configuration possible. This explains why argon is chemically inert, meaning it rarely participates in chemical reactions under normal conditions. It doesn't readily form chemical bonds with other atoms.
Argon's Properties and Applications: A Consequence of its Valence Electrons
Argon's inertness, a direct consequence of its eight valence electrons, leads to numerous applications across various fields:
1. Inert Atmosphere for Welding and Metallurgy:
Argon's lack of reactivity makes it ideal for creating an inert atmosphere during welding processes. This prevents oxidation and other unwanted chemical reactions between the molten metal and the surrounding air, leading to stronger and cleaner welds. In metallurgy, argon is used to prevent oxidation and contamination during the processing and refining of reactive metals.
2. Protective Atmosphere for Lighting and Electronics:
Argon is used in incandescent light bulbs to protect the tungsten filament from oxidation and extend the bulb's lifespan. Its inert nature prevents the filament from burning out quickly, improving the bulb's efficiency and longevity. Similarly, argon is used in some electronic devices to prevent oxidation and corrosion, ensuring optimal performance and preventing malfunctions.
3. Cryogenics:
Liquid argon, produced by cooling gaseous argon to extremely low temperatures, finds use in cryogenics. Its low boiling point and inertness make it suitable for preserving biological samples and materials requiring low-temperature storage.
4. Scientific Instruments and Analysis:
Argon's inertness makes it useful in various scientific instruments and analytical techniques. It's used as a carrier gas in gas chromatography, a technique employed to separate and analyze mixtures of volatile compounds. Its inert nature ensures that it doesn't interfere with the components being analyzed.
5. Medical Applications:
While not as prominent as some other applications, argon also finds limited use in medicine. Argon lasers are used in certain surgical procedures, taking advantage of argon's ability to selectively absorb light at certain wavelengths. This allows for precise tissue ablation and coagulation.
Argon's Position in the Periodic Table and its Relationship to Valence Electrons
Argon's position in Group 18 (or Group VIIIa) of the periodic table, the noble gases, further reinforces its unique properties. Noble gases are characterized by their complete valence shells, resulting in minimal reactivity. Their electron configurations consistently show a full octet (or duet for helium), leading to their exceptional stability.
Comparing Argon to Other Noble Gases:
The other noble gases (Helium, Neon, Krypton, Xenon, Radon) also have a full outermost electron shell. Helium, with only two electrons (1s²), follows the duet rule, while the others, like Argon, follow the octet rule. This similarity in electronic structure accounts for the shared characteristic of chemical inertness amongst the noble gases. However, the heavier noble gases (like Xenon) show slightly higher reactivity under extreme conditions due to their larger atomic size and weaker hold on their valence electrons. Argon, being intermediate in atomic size, maintains a very high level of inertness.
Beyond the Basics: Understanding Ionization Energy and Electronegativity
Two key concepts related to valence electrons are ionization energy and electronegativity. These properties further illuminate why argon is so chemically unreactive.
Ionization Energy: The Energy to Remove an Electron
Ionization energy is the energy required to remove an electron from an atom or ion in its gaseous phase. Argon has a high ionization energy because removing an electron would disrupt its stable octet, requiring a significant amount of energy. This high ionization energy contributes to argon's inertness.
Electronegativity: The Attraction for Electrons
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Argon has a very low electronegativity because its complete valence shell means it has no strong tendency to attract additional electrons. This lack of electron affinity further reinforces its inertness.
Conclusion: Argon's Inertness – A Testament to its Eight Valence Electrons
In conclusion, argon possesses eight valence electrons, forming a complete octet in its outermost energy level. This stable electron configuration is responsible for argon's remarkable chemical inertness. Its lack of reactivity, combined with its other properties, makes it a versatile and important element in various applications, ranging from welding and lighting to cryogenics and scientific instrumentation. Understanding argon's valence electron count is fundamental to appreciating its unique role in the world around us. The stable octet of argon serves as a prime example of the power of electron configuration in determining the chemical properties and applications of an element.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Sexually Transmitted Infection
Mar 25, 2025
-
168 Cm In Inches And Feet
Mar 25, 2025
-
Which Of The Following Has The Lowest Freezing Point
Mar 25, 2025
-
Biped Is To Quadruped Ostrich Is To
Mar 25, 2025
-
How Far Is Moon From Earth In Light Years
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Argon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
