How Many Valence Electrons Does Chloride Have
News Leon
Mar 18, 2025 · 6 min read
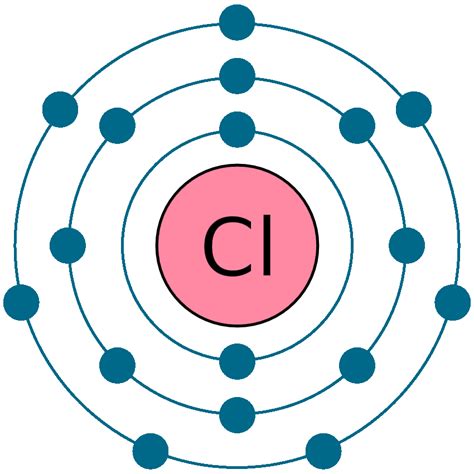
Table of Contents
How Many Valence Electrons Does Chloride Have? A Deep Dive into Atomic Structure and Chemical Bonding
Understanding the number of valence electrons an atom possesses is crucial for comprehending its chemical behavior and how it interacts with other atoms to form molecules and compounds. This article delves into the specifics of chloride (Cl⁻), explaining not only its valence electron count but also the underlying principles of atomic structure and chemical bonding that govern its properties. We'll explore the differences between chlorine atoms and chloride ions, and how this difference significantly impacts their reactivity.
Understanding Valence Electrons
Valence electrons are the electrons located in the outermost shell, or energy level, of an atom. These electrons are the ones most involved in chemical reactions and bond formation. They determine an atom's reactivity and the types of bonds it can form – whether ionic, covalent, or metallic. The number of valence electrons an atom has is largely determined by its position in the periodic table. Elements within the same group (vertical column) have the same number of valence electrons.
The Atomic Structure of Chlorine (Cl)
Chlorine (Cl), a halogen in Group 17 (VIIA) of the periodic table, has an atomic number of 17. This means a neutral chlorine atom contains 17 protons and 17 electrons. Its electronic configuration is 1s²2s²2p⁶3s²3p⁵. Let's break this down:
- 1s²: Two electrons in the first energy level (closest to the nucleus).
- 2s²2p⁶: Eight electrons in the second energy level (two in the 2s subshell and six in the 2p subshell).
- 3s²3p⁵: Seven electrons in the third energy level (two in the 3s subshell and five in the 3p subshell).
The outermost shell (the third energy level) contains seven valence electrons. This incomplete outermost shell is what makes chlorine highly reactive. Atoms strive for stability, often achieved by having a full outermost shell, resembling the electron configuration of a noble gas. Chlorine achieves this stability by gaining an electron.
The Formation of Chloride Ion (Cl⁻)
Chlorine's high reactivity stems from its desire to complete its outermost electron shell. It readily gains one electron to achieve a stable octet (eight electrons) in its valence shell. This process transforms a neutral chlorine atom into a negatively charged chloride ion (Cl⁻).
The process of gaining an electron is called reduction. During reduction, the chlorine atom accepts an electron, resulting in a change in its charge. The addition of a negatively charged electron increases the total number of negatively charged particles (electrons) exceeding the positively charged particles (protons), hence creating the -1 charge.
How Many Valence Electrons Does Chloride (Cl⁻) Have?
Once chlorine gains an electron to become a chloride ion (Cl⁻), its electron configuration becomes 1s²2s²2p⁶3s²3p⁶. Notice that the third energy level is now completely filled. Therefore, the chloride ion has eight valence electrons – a stable octet. This full valence shell makes chloride relatively unreactive compared to its neutral chlorine atom counterpart.
Chloride's Role in Chemical Bonding: Ionic Bonds
The transformation of chlorine into a chloride ion is critical to understanding ionic bonding. Ionic bonds form when one atom (like chlorine) readily gains electrons, becoming an anion (negatively charged ion), and another atom readily loses electrons, becoming a cation (positively charged ion). The electrostatic attraction between the oppositely charged ions forms the ionic bond.
For example, the reaction between sodium (Na) and chlorine (Cl) to form sodium chloride (NaCl, table salt) is a classic example of ionic bonding. Sodium (Na) readily loses one electron to achieve a stable octet, becoming a sodium ion (Na⁺). This electron is then gained by a chlorine atom, forming a chloride ion (Cl⁻). The resulting electrostatic attraction between the Na⁺ and Cl⁻ ions creates the ionic bond holding the sodium chloride crystal lattice together.
Chloride's Role in Chemical Bonding: Covalent Bonds
While chloride ions predominantly participate in ionic bonding due to their negative charge, they can also participate in interactions within covalent compounds in some scenarios. For example, certain coordination complexes can involve chloride as a ligand coordinating to a metal center. Though these interactions aren't true covalent bonds in the sense of shared electron pairs, they represent a more nuanced form of chemical bonding where chloride's electron density is influenced and interacts with the central metal ion. These interactions are often considered as interactions involving formal charge, rather than a covalent bond.
Chloride in Biological Systems
Chloride ions play vital roles in numerous biological systems. They are crucial for maintaining the balance of fluids within the body (electrolyte balance), are involved in nerve impulse transmission, and contribute to the proper functioning of many enzymes. The chloride channel, for instance, plays a critical role in regulating cell volume and maintaining the appropriate osmotic pressure within cells.
Understanding the Difference: Chlorine vs. Chloride
It's crucial to emphasize the difference between a chlorine atom and a chloride ion. While both are forms of chlorine, they have significantly different properties:
| Feature | Chlorine (Cl) | Chloride (Cl⁻) |
|---|---|---|
| Charge | Neutral (0) | Negative (-1) |
| Number of Electrons | 17 | 18 |
| Number of Valence Electrons | 7 | 8 |
| Reactivity | Highly reactive | Relatively unreactive |
| Chemical Bonding | Forms covalent bonds readily | Forms ionic bonds; participates in coordinate bonds |
Beyond the Basics: Advanced Concepts
The concept of valence electrons extends beyond simple ionic bonding. It's vital in understanding:
- Covalent bonding: Atoms share valence electrons to achieve a stable octet.
- Oxidation states: A measure of the apparent charge on an atom based on the electron sharing assumption. Chloride typically has an oxidation state of -1.
- Molecular geometry: The shape of a molecule is influenced by the arrangement of valence electrons and resulting bonding and non-bonding electron pairs.
- Polarity: Molecules can be polar or non-polar depending on how valence electrons are distributed within the molecule.
- Spectroscopy: Techniques like UV-Vis and X-ray photoelectron spectroscopy (XPS) allow us to directly probe the electronic structure and valence electron configuration of atoms and molecules.
Conclusion
In summary, a chloride ion (Cl⁻) possesses eight valence electrons, a stable octet configuration achieved by gaining one electron from a chlorine atom. This transformation significantly alters its properties, changing its charge, reactivity, and its role in chemical bonding. Understanding the number of valence electrons in chloride and its implications is fundamental to comprehending various chemical reactions and processes in diverse fields, from the inorganic chemistry of salt formation to the biochemistry of living systems. This detailed explanation provides a comprehensive understanding of chloride's atomic structure and its profound importance in chemical and biological contexts. Remember that the seemingly simple question of "how many valence electrons does chloride have?" opens the door to a rich understanding of fundamental chemical principles and the behavior of matter.
Latest Posts
Latest Posts
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Chloride Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
