How Many Valence Electrons Do Argon Have
News Leon
Mar 16, 2025 · 6 min read
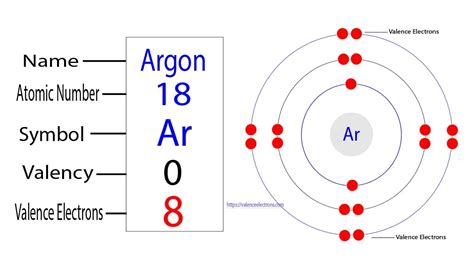
Table of Contents
- How Many Valence Electrons Do Argon Have
- Table of Contents
- How Many Valence Electrons Does Argon Have? A Deep Dive into Atomic Structure
- Understanding Valence Electrons: The Key to Chemical Bonding
- The Octet Rule: A Stable Configuration
- Argon's Position in the Periodic Table: A Clue to its Electron Configuration
- Argon's Electronic Configuration: Unveiling the Valence Electrons
- The Implications of Argon's Eight Valence Electrons
- Chemical Inertness: The Hallmark of Noble Gases
- Physical Properties: Low Reactivity and Monatomic Nature
- Applications of Argon's Inertness
- Distinguishing Valence Electrons from Other Electrons
- Conclusion: Argon and the Significance of Valence Electrons
- Latest Posts
- Latest Posts
- Related Post
How Many Valence Electrons Does Argon Have? A Deep Dive into Atomic Structure
Argon, a noble gas often overlooked in everyday life, holds a crucial place in understanding fundamental chemistry. Its unique properties, largely stemming from its electronic configuration, make it a fascinating subject of study. One key aspect that defines argon's behavior is the number of valence electrons it possesses. This article will delve deep into this question, exploring the concept of valence electrons, argon's position in the periodic table, its electronic configuration, and the implications of its electron arrangement for its chemical reactivity and physical properties.
Understanding Valence Electrons: The Key to Chemical Bonding
Before we determine the number of valence electrons in argon, let's understand the fundamental concept. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound to the nucleus and, therefore, are the ones that participate in chemical bonding. They determine an element's reactivity and the type of bonds it forms with other atoms. The number of valence electrons dictates the number of bonds an atom can potentially form. For instance, atoms strive to achieve a stable electron configuration, often resembling the noble gas configuration – a completely filled outermost shell – which is achieved through gaining, losing, or sharing valence electrons. This concept is central to understanding chemical reactions and the formation of molecules.
The Octet Rule: A Stable Configuration
The octet rule, a crucial principle in chemistry, states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell of eight valence electrons. This stable configuration, resembling that of noble gases, minimizes the atom's energy and thus increases its stability. While not universally applicable, especially for elements beyond the second period, it serves as a valuable guideline for understanding the behavior of many atoms, including those in the formation of ionic and covalent bonds. Argon, being a noble gas, already possesses this stable configuration, making it chemically inert.
Argon's Position in the Periodic Table: A Clue to its Electron Configuration
Argon (Ar) resides in Group 18 (also known as Group VIIIA or the noble gases) and Period 3 of the periodic table. The periodic table's organization reflects the recurring patterns in the electronic configuration of elements. The group number (for main group elements) typically indicates the number of valence electrons. While this rule has exceptions, it provides a useful starting point. Noble gases, including argon, are characterized by their full outermost electron shells, leading to their exceptional stability and inertness. Their position at the far right of the periodic table highlights this unique characteristic. Being in Period 3 suggests that its electrons occupy three principal energy levels or shells.
Argon's Electronic Configuration: Unveiling the Valence Electrons
The electronic configuration of an element provides a detailed description of how electrons are arranged within its atoms. Argon's atomic number is 18, indicating it has 18 protons and, in a neutral atom, 18 electrons. Its electronic configuration is written as 1s²2s²2p⁶3s²3p⁶. This notation indicates the number of electrons in each subshell.
- 1s²: Two electrons in the first energy level's s subshell.
- 2s²: Two electrons in the second energy level's s subshell.
- 2p⁶: Six electrons in the second energy level's p subshell.
- 3s²: Two electrons in the third energy level's s subshell.
- 3p⁶: Six electrons in the third energy level's p subshell.
The outermost shell for argon is the third energy level (n=3), containing both the 3s and 3p subshells. Adding the electrons in these subshells (2 + 6 = 8), we find that argon has eight valence electrons. This full octet explains argon's chemical inertness. It has no tendency to gain, lose, or share electrons to achieve a more stable configuration because it already possesses the highly stable noble gas configuration.
The Implications of Argon's Eight Valence Electrons
The presence of eight valence electrons in argon has significant implications for its physical and chemical properties:
Chemical Inertness: The Hallmark of Noble Gases
The most prominent consequence of argon's complete valence shell is its extreme chemical inertness. Unlike most elements that readily react to form compounds, argon rarely participates in chemical reactions. This is because it already possesses a stable electron configuration, and there is no energetic benefit to gaining, losing, or sharing electrons. This inertness is a defining characteristic of all noble gases.
Physical Properties: Low Reactivity and Monatomic Nature
Argon's chemical inertness directly influences its physical properties. It exists as a monatomic gas under standard conditions, meaning it doesn't form molecules with itself or other elements. Its boiling point and melting point are extremely low, reflecting the weak interatomic forces between individual argon atoms. These forces, known as van der Waals forces, are weak in noble gases due to their stable electron configurations.
Applications of Argon's Inertness
Argon's inertness makes it useful in various applications where reactivity is undesirable. Its uses include:
- Welding: Argon is used as a shielding gas in welding processes to prevent the weld metal from reacting with oxygen or nitrogen in the air, producing stronger and more consistent welds.
- Light Bulbs: It acts as a protective atmosphere within incandescent and fluorescent light bulbs, preventing the filament from oxidizing and increasing the bulb's lifespan.
- Metallurgy: Argon is used in various metallurgical processes, providing an inert atmosphere to prevent oxidation or contamination of reactive metals.
- Medical Applications: In some medical procedures, argon is used as an inert gas to displace oxygen and create a low-oxygen environment.
Distinguishing Valence Electrons from Other Electrons
It's crucial to understand that while argon has 18 electrons in total, only eight are considered valence electrons. The remaining 10 electrons are located in the inner shells and are tightly bound to the nucleus. These inner electrons do not participate in chemical bonding. The distinction between valence and inner electrons is essential for understanding an atom's chemical behavior.
Conclusion: Argon and the Significance of Valence Electrons
Argon's eight valence electrons perfectly illustrate the importance of electronic configuration in determining an element's properties. Its full outer shell leads to its chemical inertness, monatomic nature, and low reactivity, properties that are exploited in numerous industrial and medical applications. Understanding valence electrons is crucial for predicting the chemical behavior of elements and the formation of compounds. Argon, a seemingly unremarkable noble gas, serves as a powerful example of how the simple concept of valence electrons governs the complex world of chemistry. Its stable electronic structure highlights the fundamental drive of atoms to achieve stability, a principle that underpins many chemical reactions and the properties of matter itself.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Do Argon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
