How Many Valence Electrons Are There In Group 2 Elements
News Leon
Mar 30, 2025 · 6 min read
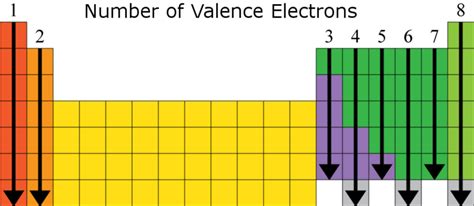
Table of Contents
How Many Valence Electrons are There in Group 2 Elements? A Deep Dive into Alkaline Earth Metals
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding an element's electron configuration is crucial to predicting its chemical behavior. This article delves into the fascinating world of Group 2 elements, also known as alkaline earth metals, focusing specifically on the number of valence electrons they possess and how this dictates their reactivity and properties.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before we dive into the specifics of Group 2, let's establish a clear understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These are the electrons that participate in chemical bonding, determining an element's reactivity and the types of compounds it can form. The number of valence electrons directly influences an element's chemical properties. Elements with similar valence electron configurations often exhibit similar chemical behaviors.
The Significance of the Outermost Shell
The outermost shell, often referred to as the valence shell, is where the action happens. Atoms strive for stability, and achieving a full valence shell, often eight electrons (the octet rule), is a primary driving force behind chemical reactions. Atoms can achieve this stability by either gaining, losing, or sharing electrons with other atoms.
Group 2 Elements: The Alkaline Earth Metals
Group 2 elements, located in the second column of the periodic table, are collectively known as alkaline earth metals. This family includes beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These metals share several characteristics due to their similar electronic configurations.
Electronic Configuration and Valence Electrons
The defining characteristic of Group 2 elements is their electronic configuration. They all have two electrons in their outermost s-orbital. This means that Group 2 elements have two valence electrons. This consistent number of valence electrons explains their similar chemical behaviors.
Example: Let's consider magnesium (Mg) as an example. Its atomic number is 12, meaning it has 12 electrons. Its electronic configuration is 1s²2s²2p⁶3s². The outermost shell is the third shell (n=3), and it contains two electrons in the 3s orbital. These two electrons are the valence electrons.
| Element | Atomic Number | Electronic Configuration | Valence Electrons |
|---|---|---|---|
| Beryllium (Be) | 4 | 1s²2s² | 2 |
| Magnesium (Mg) | 12 | 1s²2s²2p⁶3s² | 2 |
| Calcium (Ca) | 20 | 1s²2s²2p⁶3s²3p⁶4s² | 2 |
| Strontium (Sr) | 38 | 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s² | 2 |
| Barium (Ba) | 56 | 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s² | 2 |
| Radium (Ra) | 88 | [Rn]7s² | 2 |
Chemical Reactivity and the Two Valence Electrons
The two valence electrons in Group 2 elements are relatively easily lost. This is because the effective nuclear charge (the net positive charge experienced by the valence electrons) is relatively low compared to other groups. The relatively weak attraction between the nucleus and the valence electrons makes them readily available for participation in chemical bonding.
This tendency to lose two electrons results in the formation of +2 ions. This is a key characteristic of Group 2 elements, determining their chemical reactivity and the types of compounds they form. For example, magnesium readily reacts with oxygen to form magnesium oxide (MgO), losing two electrons to form the Mg²⁺ ion.
Properties Influenced by Valence Electrons
The presence of two valence electrons significantly impacts several properties of Group 2 elements:
1. Metallic Character:
Group 2 elements exhibit strong metallic character. Their ability to lose valence electrons easily contributes to their excellent electrical and thermal conductivity. The metallic bonding arising from the delocalized valence electrons is responsible for their malleability and ductility.
2. Reactivity:
Their reactivity increases as you go down the group. This is because the outermost electrons are further from the nucleus, experiencing weaker attraction, and thus are more readily lost. This increase in reactivity is reflected in the increasing ease with which they react with water and acids. For instance, Beryllium reacts very slowly, while calcium, strontium, and barium react vigorously.
3. Ionization Energy:
The ionization energy is the energy required to remove an electron from an atom. Group 2 elements have relatively low first ionization energies because losing the first valence electron is relatively easy. The second ionization energy is significantly higher because removing a second electron requires overcoming a greater electrostatic attraction.
4. Formation of Ionic Compounds:
Due to their tendency to lose two electrons, Group 2 elements primarily form ionic compounds with non-metals. The resulting ionic compounds are usually crystalline solids with high melting and boiling points. The strong electrostatic forces between the positively charged metal ions and negatively charged non-metal ions are responsible for these high melting and boiling points.
5. Formation of Covalent Compounds (Rare):
While primarily ionic, Group 2 elements can form covalent compounds under specific conditions, particularly with highly electronegative elements like oxygen. These covalent compounds are less common than their ionic counterparts.
Applications Leveraging the Unique Properties of Group 2 Elements
The unique properties stemming from their two valence electrons make Group 2 elements vital in various applications:
-
Magnesium (Mg): Widely used in lightweight alloys for automotive and aerospace industries. Also used in flash photography and as a reducing agent in chemical processes.
-
Calcium (Ca): Essential for biological processes in living organisms, acting as a structural component in bones and teeth. Also used in cement and plaster production.
-
Strontium (Sr): Used in fireworks to produce a brilliant red color. Also has applications in the production of ferrite magnets.
-
Barium (Ba): Used in various industrial applications, including the production of barium sulfate (used as a contrast agent in medical imaging) and in the manufacturing of glass and ceramics.
Conclusion: The Importance of Valence Electrons in Group 2 Chemistry
The presence of two valence electrons is the defining characteristic of Group 2 elements, dictating their chemical properties and reactivity. Understanding the electronic configuration and the significance of these valence electrons provides crucial insights into the behavior of these elements. From their metallic character to their ability to form ionic compounds, everything stems from this fundamental aspect of their atomic structure. The applications of these elements in various industries further highlight the importance of understanding and utilizing their unique properties derived directly from their two valence electrons. Further research and development in materials science and chemistry continue to explore new applications for these fascinating elements, leveraging the power and potential inherent in their unique electronic configurations.
Latest Posts
Latest Posts
-
How Many Parents Are Involved In Asexual Reproduction
Apr 01, 2025
-
What Is The Order Of The Breakdown Products Of Hemoglobin
Apr 01, 2025
-
What Muscle Subdivides The Ventral Body Cavity
Apr 01, 2025
-
How Do You Separate Iron Filings And Sand
Apr 01, 2025
-
How Many Electrons Does F Orbital Hold
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are There In Group 2 Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
