How Many Electrons Does F Orbital Hold
News Leon
Apr 01, 2025 · 5 min read
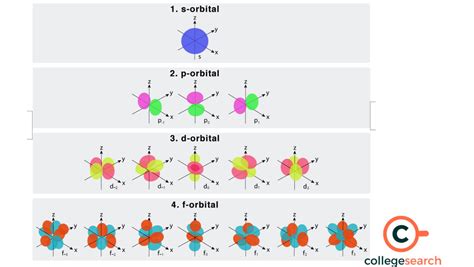
Table of Contents
How Many Electrons Does an F Orbital Hold? A Deep Dive into Atomic Structure
Understanding the electron configuration of atoms is fundamental to chemistry. A key aspect of this understanding involves comprehending the different orbitals and the number of electrons each can hold. While s, p, and d orbitals are relatively straightforward, the f orbital presents a unique challenge for many students. This article delves into the intricacies of the f orbital, explaining its capacity for electrons and its role in the periodic table.
Understanding Atomic Orbitals
Before we focus specifically on the f orbital, let's briefly review the basics of atomic orbitals. Atomic orbitals are regions of space around the nucleus of an atom where there's a high probability of finding an electron. These orbitals are characterized by different energy levels and shapes. The principal quantum number (n) determines the energy level, while the azimuthal quantum number (l) determines the shape of the orbital.
- s orbitals (l=0): These are spherical and can hold a maximum of two electrons.
- p orbitals (l=1): These are dumbbell-shaped and exist in three orientations (px, py, pz), each capable of holding two electrons, for a total of six electrons per p subshell.
- d orbitals (l=2): These have more complex shapes and exist in five orientations, allowing them to hold a total of ten electrons per d subshell.
- f orbitals (l=3): These are the most complex in shape and have seven orientations, making them capable of holding a maximum of fourteen electrons per f subshell.
The Intricacies of the F Orbital
The f orbital's complexity stems from its higher energy level and the increasing number of nodes (regions of zero electron density) within its structure. Visualizing these orbitals accurately is challenging, even with advanced computational tools. However, understanding their electron capacity is crucial for predicting the chemical behavior of elements.
The Seven f Orbitals: Shapes and Orientations
The seven f orbitals are often represented using complex mathematical equations and visualizations. While precise depiction is difficult, it's crucial to understand that they possess distinct spatial orientations. These orientations, unlike the simpler s, p, and d orbitals, don't readily lend themselves to simple geometric descriptions. The orientations are more complex and interlocked, contributing to the orbital's overall capacity.
The Significance of Electron Configuration and the Aufbau Principle
The Aufbau principle, a fundamental rule in electron configuration, dictates that electrons fill lower energy levels before occupying higher ones. Following this principle, the f orbitals are filled after the s and p orbitals of the same principal quantum number and the d orbitals of the preceding principal quantum number. This explains why the f-block elements (lanthanides and actinides) appear in a separate row at the bottom of the periodic table.
The F-Block Elements: Lanthanides and Actinides
The f orbitals are directly responsible for the properties of the f-block elements, also known as the lanthanides (elements 57-71) and actinides (elements 89-103). These elements are characterized by their similar chemical properties due to the gradual filling of the 4f and 5f orbitals, respectively. The slightly differing energy levels between the 4f and 5f orbitals and the outer s and d orbitals lead to unique behaviors in these series. This includes the lanthanide contraction, where the ionic radii of the lanthanides decrease unexpectedly across the series, affecting their chemical reactivity. Similarly, the actinide series exhibits complex chemical behavior, further influenced by relativistic effects as the nuclear charge increases significantly.
Relativistic Effects on Actinides
In the heavier actinides, relativistic effects play a significant role in their properties. Relativistic effects arise from the extremely high speeds of inner electrons, especially in heavy atoms. These effects alter the electron's mass and orbital shapes, directly impacting the chemical and physical properties. This explains why actinide chemistry is significantly more complex and diverse than lanthanide chemistry.
Beyond the Basics: Quantum Mechanics and the f Orbital
A thorough understanding of the f orbital necessitates a deeper exploration of quantum mechanics. The Schrödinger equation, a cornerstone of quantum mechanics, provides a mathematical framework for describing the behavior of electrons in atoms. Solving this equation for f orbitals yields complex wave functions that determine the probability density of finding electrons in a specific region of space.
Wave Functions and Probability Density
The wave functions for f orbitals are significantly more complex than those for s, p, and d orbitals. These complex functions are difficult to visualize directly, but the resulting probability density provides a better understanding of the electron's spatial distribution. This probability density highlights the regions where an electron is most likely to be found, confirming the intricate shape and orientation described earlier.
Practical Applications: The Importance of Understanding F Orbitals
The understanding of f orbitals and their electron capacity is not confined to theoretical concepts. It has numerous practical applications across various scientific disciplines:
- Material Science: The properties of f-block elements are crucial in designing advanced materials with specific magnetic, catalytic, and optical properties. The unique electronic structure of these elements is utilized in the development of high-performance magnets, catalysts for industrial processes, and specialized optical devices.
- Nuclear Chemistry and Physics: The actinide series plays a significant role in nuclear reactions and technologies. Understanding their electronic structure is vital for nuclear power generation, nuclear medicine, and managing nuclear waste.
- Catalysis: Lanthanide and actinide compounds serve as effective catalysts in a wide range of chemical reactions. Their variable oxidation states and electronic configurations make them particularly suitable for promoting chemical transformations.
Conclusion: The F Orbital and its Significance
The f orbital, although complex and often challenging to visualize, holds a significant place in our understanding of atomic structure and chemical behavior. Its capacity to hold fourteen electrons is directly responsible for the properties of the lanthanides and actinides, elements with crucial roles in material science, nuclear technologies, and catalysis. A robust grasp of the f orbital's properties is essential for advancements across various scientific fields, showcasing its profound relevance beyond academic study. Further research continues to refine our understanding of relativistic effects and the nuanced interplay of electrons within these complex orbitals, paving the way for future breakthroughs.
Latest Posts
Latest Posts
-
Calculate The Binding Energy Per Nucleon
Apr 02, 2025
-
Oxidation Number Of Iron In Fe3o4
Apr 02, 2025
-
How Many Sig Figs Are In 0 020
Apr 02, 2025
-
Which Of The Following Companies Is A Manufacturer Of Cpus
Apr 02, 2025
-
Greatest Common Factor Of 8 And 36
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does F Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
