How Many Valence Electrons Are In Zinc
News Leon
Mar 14, 2025 · 5 min read
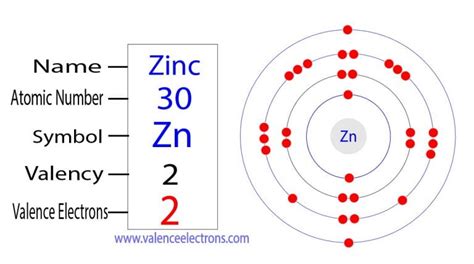
Table of Contents
How Many Valence Electrons Are in Zinc? A Deep Dive into Zinc's Electronic Structure
Zinc, a ubiquitous element found in everything from pennies to crucial enzymes, plays a vital role in various biological and industrial processes. Understanding its electronic structure, particularly the number of valence electrons, is crucial to grasping its chemical behavior and reactivity. This comprehensive article will delve into the specifics of zinc's electron configuration, explaining how to determine its valence electrons and exploring the implications of this number for its properties.
Understanding Valence Electrons: The Key to Reactivity
Before we jump into the specifics of zinc, let's establish a solid understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (energy level) of an atom. These electrons are the ones most involved in chemical bonding, determining an element's reactivity and the types of compounds it can form. The number of valence electrons directly influences an element's properties, including its electronegativity, ionization energy, and bonding preferences.
Think of valence electrons as the atom's "social butterflies" – they are the ones interacting with other atoms to form relationships (chemical bonds). The inner electrons, closer to the nucleus, are held more tightly and are less involved in these interactions.
Determining Zinc's Electron Configuration
To determine the number of valence electrons in zinc (Zn), we need to examine its electron configuration. Zinc has an atomic number of 30, meaning it possesses 30 protons and, in a neutral atom, 30 electrons. The electron configuration, which describes how these electrons are distributed among the different energy levels and sublevels, is determined using the Aufbau principle and Hund's rule.
The electron configuration of zinc is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰.
Let's break this down:
- 1s²: Two electrons occupy the first energy level (n=1), in the s sublevel.
- 2s² 2p⁶: Eight electrons occupy the second energy level (n=2), with two in the s sublevel and six in the p sublevel.
- 3s² 3p⁶: Eight electrons occupy the third energy level (n=3), with two in the s sublevel and six in the p sublevel.
- 4s² 3d¹⁰: Ten electrons occupy the fourth energy level (n=4), with two in the s sublevel and ten in the d sublevel. Note that the 3d sublevel fills after the 4s sublevel according to the Aufbau principle.
Identifying the Valence Electrons in Zinc
Now comes the crucial part: identifying the valence electrons. While the simple definition points to the outermost shell, the situation with transition metals like zinc is slightly more nuanced. The outermost shell for zinc is the fourth energy level (n=4), containing the 4s² electrons. However, the 3d¹⁰ electrons, while not strictly in the outermost shell, also participate in chemical bonding, albeit less readily than the 4s electrons.
Therefore, the generally accepted number of valence electrons in zinc is two. This is because the 4s electrons are primarily involved in chemical bonding, while the 3d electrons are considered relatively "inner" and less reactive. The filled d-shell contributes to the relative inertness of zinc. While the 3d electrons can participate under certain conditions, their influence is less significant compared to the 4s electrons in typical chemical reactions.
The Implications of Zinc's Two Valence Electrons
The fact that zinc possesses only two valence electrons has significant implications for its chemical behavior:
-
Limited Reactivity: Compared to elements with more valence electrons, zinc is relatively unreactive. Its filled d-shell contributes to this low reactivity, creating a stable electron configuration that resists readily losing or sharing electrons.
-
Formation of +2 Ions: Zinc readily loses its two valence electrons to form a +2 ion (Zn²⁺). This is its most common oxidation state. The formation of this stable ion explains zinc's prevalence in ionic compounds.
-
Coordination Chemistry: While not highly reactive, zinc readily forms coordination complexes. These complexes involve zinc ions bonding to ligands (molecules or ions) through coordinate covalent bonds. The relatively small size and +2 charge of the zinc ion makes it a favorable metal for such coordination chemistry.
Zinc in Biological Systems: The Role of Valence Electrons
Zinc's properties are essential for its crucial role in various biological systems. Enzymes called zinc metalloenzymes rely heavily on zinc ions. The two valence electrons lost to form Zn²⁺ allow it to act as a Lewis acid, accepting electron pairs from ligands within the enzyme's active site. This Lewis acidity facilitates various catalytic functions within these enzymes, influencing crucial processes in the body.
For instance, carbonic anhydrase, an enzyme vital for carbon dioxide transport and pH regulation, uses a zinc ion in its catalytic center. The zinc ion's ability to coordinate with water molecules and bicarbonate ions is directly related to its +2 charge and its capacity to participate in coordination chemistry.
Zinc in Industrial Applications: From Galvanization to Alloys
Zinc's properties, determined by its two valence electrons, are exploited extensively in various industrial applications:
-
Galvanization: Zinc's resistance to corrosion makes it an excellent protective coating for steel. The process of galvanization involves coating steel with zinc to prevent rust. Zinc's low reactivity protects the underlying steel from oxidation.
-
Alloys: Zinc is a component in numerous alloys, enhancing their properties. Brass, an alloy of copper and zinc, is renowned for its strength, durability, and corrosion resistance. These properties stem directly from the electronic structure of zinc and its ability to form metallic bonds within the alloy.
-
Batteries: Zinc is a key component in various battery systems, such as dry cells and alkaline batteries. Zinc's ability to readily lose its two valence electrons facilitates the electron transfer that powers these batteries.
Conclusion: The Significance of Two Valence Electrons
The seemingly simple number of two valence electrons in zinc holds profound implications for its behavior, both in biological and industrial contexts. Understanding zinc's electron configuration provides a foundation for comprehending its low reactivity, its propensity for forming +2 ions, and its capacity to participate in coordination chemistry. These properties are directly responsible for zinc's vital role in biological processes and its extensive use in various industrial applications. From protecting steel against rust to facilitating enzyme function, the influence of zinc's two valence electrons is undeniable. Further research continues to unveil the full extent of zinc's importance across various scientific fields. The next time you encounter zinc, remember the significance of those two crucial valence electrons!
Latest Posts
Latest Posts
-
What Is The Ultimate Goal Of A Political Party
Mar 14, 2025
-
Which Chamber Of The Heart Has The Thickest Muscular Wall
Mar 14, 2025
-
The Product Of Two Irrational Numbers Is Irrational
Mar 14, 2025
-
Which Element Is Found In All Organic Compounds
Mar 14, 2025
-
What Is The Largest Single Mass Of Lymphatic Tissue
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Zinc . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
