How Many Valence Electrons Are In Na
News Leon
Mar 18, 2025 · 5 min read
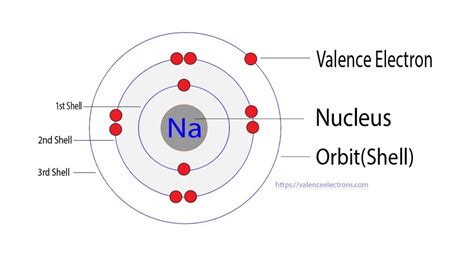
Table of Contents
How Many Valence Electrons Are in Na? Understanding Sodium's Reactivity
Sodium (Na), a highly reactive alkali metal, plays a crucial role in various biological and industrial processes. Understanding its chemical behavior hinges on grasping its electron configuration, specifically the number of valence electrons. This article delves deep into the electronic structure of sodium, explaining how to determine its valence electrons, and exploring the implications of this number for its reactivity and bonding characteristics.
Understanding Valence Electrons
Before we dive into the specifics of sodium, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons dictates how many bonds an atom can typically form.
Determining the number of valence electrons is crucial for predicting chemical behavior. It allows us to understand why some elements are highly reactive, while others are inert. This knowledge underpins our understanding of chemical reactions and the formation of molecules and compounds.
Electron Configuration of Sodium
To determine the number of valence electrons in sodium, we need to examine its electron configuration. Sodium's atomic number is 11, meaning it has 11 protons and 11 electrons in a neutral atom. The electron configuration describes how these electrons are distributed among different energy levels (shells) and sublevels (orbitals).
The electron configuration of sodium is 1s²2s²2p⁶3s¹.
Let's break this down:
- 1s²: Two electrons occupy the first energy level (shell), specifically the 's' subshell.
- 2s²: Two electrons occupy the second energy level, 's' subshell.
- 2p⁶: Six electrons occupy the second energy level, 'p' subshell. The 'p' subshell can hold a maximum of six electrons.
- 3s¹: One electron occupies the third energy level, 's' subshell.
Identifying Valence Electrons in Sodium
The outermost shell of an atom is the one with the highest principal quantum number (n). In sodium's electron configuration, the highest principal quantum number is 3. Therefore, the outermost shell is the third energy level.
The third energy level contains only one electron, residing in the 3s orbital. This means that sodium has one valence electron.
Implications of One Valence Electron
The presence of only one valence electron has profound implications for sodium's chemical behavior:
-
High Reactivity: Sodium readily loses its single valence electron to achieve a stable, filled outermost shell (like the noble gas neon). This makes it highly reactive, especially with nonmetals that readily accept electrons.
-
Ionic Bonding: Sodium typically forms ionic bonds by losing its valence electron to a more electronegative atom, forming a positively charged ion (Na⁺). This positive ion is then attracted to the negatively charged ion of the other atom, resulting in an ionic compound. For example, sodium reacts vigorously with chlorine (Cl) to form sodium chloride (NaCl), common table salt, through the transfer of sodium's valence electron to chlorine.
-
Metallic Bonding: Sodium also exhibits metallic bonding in its pure metallic state. The valence electrons are delocalized, forming a "sea" of electrons that surrounds the positively charged sodium ions. This "sea" of electrons allows for the excellent electrical and thermal conductivity of sodium metal.
Sodium's Reactions: A Closer Look
The single valence electron dictates the nature of sodium's reactions. Let's examine some examples:
-
Reaction with Water: Sodium reacts explosively with water, producing hydrogen gas and sodium hydroxide. The single valence electron is transferred to water molecules, breaking the water molecules and forming hydrogen gas (H₂) and hydroxide ions (OH⁻). The reaction is highly exothermic, releasing significant heat.
-
Reaction with Halogens: Sodium reacts violently with halogens (e.g., chlorine, bromine, iodine) to form ionic compounds known as halides (NaCl, NaBr, NaI). The single valence electron is readily transferred to the halogen atom, forming a stable ionic bond.
-
Reaction with Oxygen: Sodium reacts slowly with oxygen in the air to form sodium oxide (Na₂O). This reaction is less vigorous than its reactions with water or halogens.
Comparison with Other Alkali Metals
Sodium belongs to Group 1 of the periodic table, known as the alkali metals. All alkali metals have one valence electron and exhibit similar chemical properties, although the reactivity increases as you move down the group. Lithium (Li), for example, also has one valence electron, but it is less reactive than sodium due to its smaller atomic size and stronger hold on its valence electron. Potassium (K), rubidium (Rb), and cesium (Cs) are even more reactive than sodium.
Applications of Sodium and its Compounds
The unique properties stemming from its single valence electron make sodium and its compounds vital in numerous applications:
-
Sodium Chloride (NaCl): Widely used as table salt, in food preservation, and in various industrial processes.
-
Sodium Hydroxide (NaOH): A strong base used in soap making, paper production, and drain cleaners.
-
Sodium Carbonate (Na₂CO₃): Used in glass manufacturing, water softening, and detergents.
-
Sodium Lamps: Used in street lighting and other applications due to their bright yellow-orange light emission.
-
Coolant in Nuclear Reactors: Liquid sodium is used as a coolant in some nuclear reactors due to its excellent heat transfer properties.
Safety Precautions when Handling Sodium
Because of its high reactivity, sodium metal requires careful handling. It should be stored under oil or inert atmosphere to prevent reaction with air and moisture. Direct contact with water should be avoided due to the risk of explosion. Appropriate safety goggles and gloves should always be used when handling sodium.
Conclusion: The Significance of One Valence Electron
The single valence electron of sodium is the key to understanding its reactivity and diverse applications. This electron's tendency to be lost to achieve a stable electron configuration drives sodium's participation in various chemical reactions, resulting in the formation of ionic compounds and its contribution to essential industrial and biological processes. Understanding the electron configuration and the implications of valence electrons is fundamental to comprehending chemical behavior and reactivity across the periodic table. The case of sodium perfectly illustrates this principle, highlighting the importance of valence electrons in shaping the properties and applications of an element.
Latest Posts
Latest Posts
-
Why Is Boiling Water A Physical Change
Mar 19, 2025
-
What Is The Product Of The Following Sequence Of Reactions
Mar 19, 2025
-
How Are Weathering And Erosion Difference
Mar 19, 2025
-
Double Replacement Examples In Real Life
Mar 19, 2025
-
The Study Of Population Is Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Na . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
