How Many Valence Electrons Are In Bromine
News Leon
Mar 17, 2025 · 4 min read
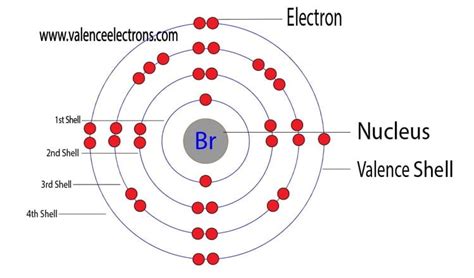
Table of Contents
How Many Valence Electrons Are in Bromine? A Deep Dive into Atomic Structure
Bromine, a fascinating element with a rich history and diverse applications, holds a unique position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is crucial to comprehending its chemical behavior and reactivity. This article delves deep into the question: how many valence electrons are in bromine? We'll explore the concept of valence electrons, examine bromine's position in the periodic table, and explain how its electronic configuration determines its reactivity. We'll also explore the applications of bromine and its compounds, showcasing the practical implications of its valence electron count.
Understanding Valence Electrons
Before we delve into the specifics of bromine, let's establish a solid foundation. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound to the nucleus and, therefore, are primarily responsible for an atom's chemical properties and its ability to form chemical bonds with other atoms. They determine an element's reactivity, its tendency to gain, lose, or share electrons to achieve a stable electron configuration, usually a full outer shell (often eight electrons, following the octet rule).
The number of valence electrons an atom possesses is directly related to its position in the periodic table. This is one of the reasons the periodic table is such a powerful tool for chemists. The group number (vertical column) generally indicates the number of valence electrons for main group elements (groups 1-18). Transition metals, however, have a more complex valence electron structure.
Bromine's Position and Electronic Configuration
Bromine (Br) is a nonmetal located in Group 17 (also known as Group VIIA or the halogens) of the periodic table. Its atomic number is 35, meaning it has 35 protons and 35 electrons in a neutral atom. To understand its valence electrons, we need to examine its electronic configuration.
The electronic configuration of bromine is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁵.
This configuration tells us the distribution of electrons across different energy levels (shells) and subshells within the atom. The outermost shell is the fourth shell (n=4), which contains the 4s and 4p sublevels. Let's break it down:
- 4s²: Two electrons in the 4s subshell.
- 4p⁵: Five electrons in the 4p subshell.
Therefore, bromine has a total of 2 + 5 = 7 valence electrons.
Why 7 Valence Electrons Matter for Bromine's Reactivity
Having seven valence electrons makes bromine highly reactive. To achieve a stable octet (eight electrons in its outermost shell), bromine readily gains one electron, forming a bromide ion (Br⁻) with a full outer shell (3s²3p⁶4s²3d¹⁰4p⁶). This tendency to gain an electron explains why bromine is a strong oxidizing agent. It readily accepts electrons from other atoms or molecules, causing them to be oxidized.
Bromine's Chemical Behavior and Compound Formation
Bromine's seven valence electrons directly impact the types of compounds it forms. It readily forms ionic compounds with metals, where it gains an electron to become a bromide ion. For example, sodium bromide (NaBr) is formed when sodium (Na) readily loses one electron to bromine.
Bromine can also form covalent bonds with other nonmetals by sharing electrons to achieve a stable octet. For instance, hydrogen bromide (HBr) is a covalent compound where bromine shares one electron with hydrogen.
Applications of Bromine and its Compounds
The unique chemical properties stemming from its seven valence electrons have led to numerous applications for bromine and its compounds. These include:
-
Flame retardants: Organobromine compounds are used extensively as flame retardants in various materials, such as plastics and textiles, due to their ability to interfere with the combustion process.
-
Water purification: Bromine and its compounds are effective disinfectants and are used in water treatment to kill bacteria and other microorganisms.
-
Agricultural chemicals: Certain bromine-containing compounds are used as fumigants and pesticides in agriculture.
-
Pharmaceuticals: Bromine-containing compounds find applications in certain pharmaceuticals, although their use is becoming less prevalent due to environmental concerns.
-
Photography: Bromine compounds were historically used in photography, though they are largely replaced by other methods.
Environmental Considerations
While bromine and its compounds have many useful applications, it's crucial to acknowledge environmental considerations. Some organobromine compounds are persistent organic pollutants (POPs), meaning they persist in the environment and can accumulate in living organisms, potentially leading to harmful effects. Strict regulations are in place to control the production and use of these compounds.
Conclusion: The Significance of Seven Valence Electrons
In summary, bromine possesses seven valence electrons, a feature that fundamentally dictates its chemical behavior and reactivity. This number explains its strong tendency to gain one electron to achieve a stable octet, leading to the formation of bromide ions and a wide range of compounds with diverse applications. Understanding the significance of valence electrons is key to appreciating the properties and applications of bromine and its compounds. While incredibly useful, the environmental impact of certain bromine compounds necessitates careful management and the ongoing development of more sustainable alternatives. Future research will undoubtedly continue to explore both the beneficial uses and environmental considerations related to this fascinating element and its unique electronic configuration.
Latest Posts
Latest Posts
-
Predict What Is Present In Each Of The Following
Mar 18, 2025
-
Which Of The Following Word Is Different From The Others
Mar 18, 2025
-
Reverse List Python Without Inbuilt Function
Mar 18, 2025
-
A Current Of One Ampere Is Passed Through
Mar 18, 2025
-
Difference Between Earthing And Grounding And Neutral
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Bromine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
