How Many Valence Electrons Are In Ar
News Leon
Mar 18, 2025 · 6 min read
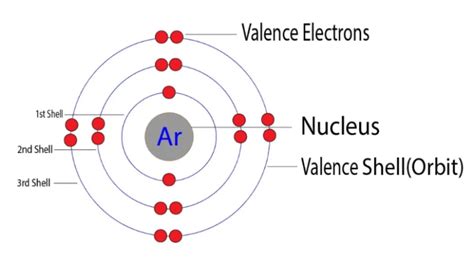
Table of Contents
How Many Valence Electrons Are in Argon? A Deep Dive into Atomic Structure and Chemical Behavior
Argon (Ar), a noble gas residing in Group 18 of the periodic table, is renowned for its chemical inertness. This inertness stems directly from its electronic configuration, specifically the number of valence electrons it possesses. Understanding this number is key to grasping Argon's properties and its role in various applications. This article will delve into the details, explaining not just how many valence electrons Argon has, but why this number is so significant.
Understanding Valence Electrons: The Key to Reactivity
Before focusing on Argon specifically, let's establish a foundational understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. These electrons are the primary players in chemical bonding – the forces that hold atoms together to form molecules and compounds. Atoms strive for stability, often achieved by having a full valence shell, typically containing eight electrons (the octet rule, with some exceptions). This drive for stability dictates how atoms interact and form chemical bonds.
The Role of Electron Shells and Subshells
Electrons don't just randomly orbit the nucleus. They are arranged in specific energy levels or shells, each capable of holding a maximum number of electrons. These shells are further subdivided into subshells (s, p, d, f), each with its own electron capacity. The filling of these shells and subshells follows specific rules, dictated by the Aufbau principle and Hund's rule, leading to the unique electronic configuration for each element.
The first shell (n=1) can hold a maximum of two electrons in the 1s subshell. The second shell (n=2) can hold up to eight electrons (2 in the 2s subshell and 6 in the 2p subshell). The third shell (n=3) can accommodate up to 18 electrons (2 in 3s, 6 in 3p, and 10 in 3d), and so on. The outermost shell containing electrons is the valence shell, and the electrons within this shell are the valence electrons.
Argon's Electronic Configuration: Unveiling the 8 Valence Electrons
Argon's atomic number is 18, meaning it has 18 protons and 18 electrons in a neutral atom. Its electronic configuration is written as 1s²2s²2p⁶3s²3p⁶. Let's break this down:
- 1s²: Two electrons fill the first shell's s subshell.
- 2s²: Two electrons fill the second shell's s subshell.
- 2p⁶: Six electrons fill the second shell's p subshell.
- 3s²: Two electrons fill the third shell's s subshell.
- 3p⁶: Six electrons fill the third shell's p subshell.
The third shell, containing both the 3s and 3p subshells, is Argon's outermost shell, also its valence shell. Adding the electrons in the 3s and 3p subshells (2 + 6 = 8), we find that Argon has 8 valence electrons.
The Significance of a Full Valence Shell
This full valence shell is crucial to understanding Argon's chemical behavior. A full valence shell represents a state of maximum stability. Atoms with full valence shells, like Argon, are exceptionally unreactive because they have no tendency to gain, lose, or share electrons to achieve a more stable configuration. This is why Argon, and other noble gases, are considered inert.
Argon's Inertness: A Consequence of its Electronic Structure
Argon's inertness is not merely a theoretical concept; it's a demonstrable fact reflected in its properties and applications. Its lack of reactivity means it doesn't readily form chemical bonds with other atoms. This inertness has led to its widespread use in various applications where preventing unwanted chemical reactions is vital.
Applications Leveraging Argon's Inertness
The following examples highlight how Argon's unique electronic structure translates into practical applications:
- Welding: Argon's inertness protects the weld from atmospheric gases (like oxygen and nitrogen) that can contaminate the weld metal, compromising its strength and quality.
- Light Bulbs: Argon is used as a filler gas in incandescent light bulbs to prevent the filament from oxidizing and burning out prematurely.
- Laser Technology: Argon ion lasers produce highly intense and monochromatic light, used in various applications including medical procedures, scientific research, and industrial processes.
- Metallurgy: Argon is used in metallurgy to prevent oxidation and contamination during processes like arc welding and metal casting.
- Healthcare: Argon is used in some medical applications, leveraging its inertness for its non-reactive nature.
- Scientific Research: Due to its inert nature, Argon is used in various scientific experiments that require a controlled and non-reactive environment.
Comparing Argon's Valence Electrons to Other Elements
Comparing Argon's valence electrons to those of other elements helps further illuminate its unique chemical behavior.
Noble Gases: A Family of Inert Elements
All noble gases (Helium, Neon, Argon, Krypton, Xenon, Radon) share a common characteristic: they have a full valence shell. Helium, with two electrons in its single shell, is an exception to the octet rule, but it also has a full valence shell. This full valence shell is the reason for their exceptional inertness.
Other Elements: Varying Reactivity
Elements in other groups of the periodic table have incomplete valence shells, leading to varying degrees of reactivity. For example, alkali metals (Group 1) have only one valence electron, making them highly reactive as they readily lose this electron to achieve a stable configuration. Halogens (Group 17) have seven valence electrons and readily gain one electron to complete their octet. Transition metals display more complex behavior due to the involvement of d electrons.
Beyond the Basics: Advanced Concepts Related to Argon's Valence Electrons
While understanding Argon’s 8 valence electrons provides a solid foundation, more nuanced concepts related to its electronic structure exist:
Ionization Energy and Electron Affinity
Argon's high ionization energy (the energy required to remove an electron) reflects its stability. Removing an electron from its full valence shell requires significant energy. Similarly, Argon's electron affinity (the energy change associated with gaining an electron) is very low, as adding an electron would disrupt its stable, full valence shell.
Excited States and Spectral Lines
While Argon is generally inert, it can be excited by absorbing energy. This causes an electron to jump to a higher energy level, which is unstable. When the electron returns to its ground state, it releases energy as light, producing characteristic spectral lines used for Argon identification.
Argon Isotopes and Nuclear Properties
While the number of valence electrons defines Argon's chemical behavior, its isotopes (variations in neutron number) affect its nuclear properties. These differences can influence applications involving radioactive isotopes.
Conclusion: The Crucial Role of Valence Electrons in Defining Argon
The number of valence electrons in Argon — eight — is not just a numerical fact; it's the fundamental reason for Argon's chemical inertness and its diverse applications. Its full valence shell provides exceptional stability, leading to a lack of reactivity that is exploited across various industries and scientific endeavors. Understanding this core concept is essential for comprehending the behavior of Argon and its unique position in the periodic table. The exploration of Argon's electronic structure opens doors to understanding atomic behavior, chemical bonding, and the practical applications stemming from the unique properties of elements.
Latest Posts
Latest Posts
-
Why Is Boiling Water A Physical Change
Mar 19, 2025
-
What Is The Product Of The Following Sequence Of Reactions
Mar 19, 2025
-
How Are Weathering And Erosion Difference
Mar 19, 2025
-
Double Replacement Examples In Real Life
Mar 19, 2025
-
The Study Of Population Is Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Ar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
