How Many Unpaired Electrons Does Phosphorus Have
News Leon
Mar 15, 2025 · 6 min read
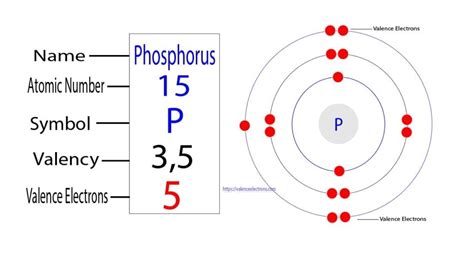
Table of Contents
How Many Unpaired Electrons Does Phosphorus Have? A Deep Dive into Electron Configuration and its Implications
Phosphorus, a crucial element in biological systems and industrial processes, presents an intriguing case study in electron configuration. Understanding its electronic structure is key to comprehending its reactivity and diverse applications. This article will delve deep into the question: How many unpaired electrons does phosphorus have? We'll explore its electron configuration, orbital diagrams, and the implications of its unpaired electrons on its chemical behavior.
Understanding Electron Configuration
Before we tackle the specific case of phosphorus, let's establish a foundational understanding of electron configuration. This refers to the arrangement of electrons in the various energy levels and sublevels within an atom. These arrangements are governed by the principles of quantum mechanics, specifically the Pauli Exclusion Principle (which states that no two electrons can have the same set of four quantum numbers) and Hund's Rule (which states that electrons will individually occupy each orbital within a subshell before doubling up).
Electrons fill orbitals in order of increasing energy, following the Aufbau principle. This means lower energy levels fill first, with the filling order generally following the diagram: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on. Each orbital can hold a maximum of two electrons, and these electrons have opposite spins (represented by ↑ and ↓).
Determining the Electron Configuration of Phosphorus
Phosphorus (P) has an atomic number of 15, meaning it has 15 protons and 15 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle and fill the orbitals according to their energy levels:
- 1s²: The first energy level (n=1) has only one subshell, the 's' subshell, which can hold up to two electrons. Phosphorus fills this subshell completely.
- 2s²: The second energy level (n=2) begins with the 's' subshell, which again holds two electrons. This is also filled completely.
- 2p⁶: The second energy level also contains the 'p' subshell, which can hold up to six electrons (three orbitals, each holding two electrons). Phosphorus fills this subshell completely.
- 3s²: The third energy level (n=3) starts with the 's' subshell, holding another two electrons.
- 3p³: Finally, we reach the 'p' subshell of the third energy level. This subshell has three orbitals, and phosphorus has three electrons remaining. According to Hund's Rule, these three electrons will each occupy a separate orbital before pairing up.
Therefore, the complete electron configuration of phosphorus is 1s²2s²2p⁶3s²3p³.
Visualizing with Orbital Diagrams
Orbital diagrams provide a visual representation of electron configuration. Each orbital is represented by a box, and electrons are represented by arrows. The diagram for phosphorus's 3p subshell, which holds the unpaired electrons, would look like this:
3p: ↑ ↑ ↑
The 1s, 2s, 2p, and 3s orbitals are all filled with paired electrons (↑↓), but the 3p subshell contains three unpaired electrons, each occupying a separate orbital.
The Significance of Unpaired Electrons
The presence of unpaired electrons has significant implications for the chemical behavior of phosphorus:
-
Paramagnetism: Substances with unpaired electrons are paramagnetic, meaning they are weakly attracted to a magnetic field. This is because the unpaired electrons' spins create a net magnetic moment. Phosphorus's paramagnetism is a direct consequence of its three unpaired electrons.
-
Reactivity: Unpaired electrons make an atom more reactive. These electrons readily participate in chemical bonding to achieve a more stable electron configuration (often by forming pairs). This explains why phosphorus readily forms compounds with other elements, such as oxygen (in phosphorus oxides) and chlorine (in phosphorus chlorides).
-
Formation of Bonds: Phosphorus's three unpaired electrons allow it to form three covalent bonds with other atoms. For instance, in PH₃ (phosphine), phosphorus forms three single covalent bonds with three hydrogen atoms. In PCl₃ (phosphorus trichloride), it forms three single covalent bonds with three chlorine atoms. Phosphorus can also form multiple bonds under certain circumstances, demonstrating its versatility in bonding.
Phosphorus's allotropes and unpaired electrons
Phosphorus exists in several allotropic forms, meaning it can exist in different structural modifications. White phosphorus, the most reactive form, consists of P₄ tetrahedra, where each phosphorus atom is bonded to three other phosphorus atoms. Even in this structure, the electronic configuration of each phosphorus atom still has those three unpaired electrons contributing to its high reactivity. The different allotropes of phosphorus exhibit varying degrees of reactivity due to differences in bonding and arrangement but the fundamental electron configuration with unpaired electrons remains influential.
Phosphorus in Biological Systems and Industrial Applications
The reactivity of phosphorus, directly tied to its unpaired electrons, is fundamental to its significance in both biological and industrial contexts.
Biological Significance:
-
DNA and RNA: Phosphorus is a critical component of DNA and RNA, the molecules that carry genetic information. The phosphate groups in the backbone of these molecules are crucial for their structural integrity and function.
-
ATP: Adenosine triphosphate (ATP) is the primary energy currency of cells. The phosphate bonds in ATP store significant energy, which is released when these bonds are broken. Phosphorus's ability to form strong and energy-rich bonds is key to this process.
-
Phospholipids: Phospholipids are essential components of cell membranes, forming a lipid bilayer that separates the cell's interior from its surroundings.
Industrial Applications:
-
Fertilizers: Phosphorus is a major component of fertilizers, crucial for plant growth and agricultural productivity. Its role in the formation of vital biological molecules within plants directly relates to its electron configuration and bonding capabilities.
-
Detergents: Phosphates were commonly used in detergents, though their use has been reduced due to environmental concerns. Their function in detergents related to their ability to soften water.
-
Matchsticks: White phosphorus was historically used in the manufacturing of matchsticks, although its high toxicity has led to its replacement with other chemicals. Its reactivity, related to unpaired electrons, was utilized for its ignition properties.
-
Semiconductors: Phosphorus is used in the semiconductor industry as a dopant in silicon, altering its electrical properties. This shows the influence of its electronic structure even at the microscopic level, leading to macroscopic technological developments.
Conclusion: The Unpaired Electrons Define Phosphorus
The answer to the question, "How many unpaired electrons does phosphorus have?" is definitively three. These unpaired electrons in the 3p subshell are not merely an abstract detail; they are the driving force behind phosphorus's remarkable reactivity, its diverse bonding patterns, and ultimately, its crucial role in both biological systems and numerous industrial processes. Understanding phosphorus's electron configuration is crucial to grasping its fundamental properties and its multifaceted significance in our world. Its paramagnetism, its ability to form strong bonds, and its overall chemical behavior are all direct consequences of this unique electronic structure. From the genetic code to fertilizer production, the influence of phosphorus's three unpaired electrons is undeniably profound.
Latest Posts
Latest Posts
-
A Negatively Charged Ion Is Called
Mar 15, 2025
-
Two Different Isotopes Of An Element Have Different
Mar 15, 2025
-
If A Pea Plant Shows A Recessive Phenotype
Mar 15, 2025
-
Is A Patent A Current Asset
Mar 15, 2025
-
Why Are Producers So Important To An Ecosystem
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Unpaired Electrons Does Phosphorus Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
