How Many Unpaired Electrons Does Nitrogen Have
News Leon
Mar 29, 2025 · 5 min read
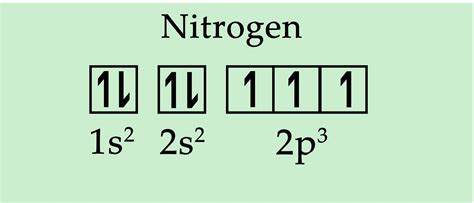
Table of Contents
How Many Unpaired Electrons Does Nitrogen Have? A Deep Dive into Atomic Structure and Electronic Configuration
Nitrogen, a ubiquitous element crucial to life as we know it, possesses a fascinating electronic structure that dictates its chemical behavior. Understanding the number of unpaired electrons in nitrogen is key to unraveling its reactivity and the properties of the numerous compounds it forms. This article delves into the atomic structure of nitrogen, explains how to determine its electron configuration, and ultimately answers the central question: how many unpaired electrons does nitrogen have?
Understanding Atomic Structure and Electron Configuration
Before tackling the specifics of nitrogen, let's establish a foundational understanding of atomic structure. Atoms are composed of a nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons occupy specific energy levels or shells, and within each shell, they are further organized into subshells (s, p, d, f). The arrangement of electrons in these shells and subshells is described by the electron configuration. This configuration dictates an atom's chemical properties and reactivity. Electrons fill orbitals according to the Aufbau principle (filling lower energy levels first), Hund's rule (maximizing unpaired electrons in a subshell before pairing), and the Pauli exclusion principle (no two electrons can have the same four quantum numbers).
The Significance of Unpaired Electrons
Unpaired electrons are electrons that occupy an orbital alone, rather than being paired with another electron in the same orbital. These unpaired electrons are responsible for the magnetic properties of an atom or molecule, as well as its ability to form chemical bonds. They are highly reactive, readily participating in chemical reactions to achieve a more stable, lower energy state. Atoms strive to achieve a full outer electron shell (octet rule for most elements), either by gaining, losing, or sharing electrons.
Determining Nitrogen's Electron Configuration
Nitrogen (N) has an atomic number of 7, meaning it has 7 protons and 7 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle and fill the electron shells sequentially:
- 1s²: The first energy level (shell) contains the 1s subshell, which can hold up to two electrons. These two electrons fill the 1s orbital.
- 2s²: The second energy level contains the 2s and 2p subshells. The 2s subshell can also hold two electrons, filling the 2s orbital.
- 2p³: This leaves us with three remaining electrons. The 2p subshell has three orbitals (2px, 2py, 2pz), each capable of holding two electrons. According to Hund's rule, these three electrons will each occupy a separate 2p orbital individually before pairing up.
Therefore, the complete electron configuration of nitrogen is 1s²2s²2p³.
How Many Unpaired Electrons Does Nitrogen Have?
Based on the electron configuration (1s²2s²2p³), we can definitively answer the central question: nitrogen has three unpaired electrons. These three unpaired electrons reside in the three individual 2p orbitals (2px, 2py, 2pz). This fact is crucial for understanding nitrogen's chemical behavior.
Nitrogen's Reactivity and its Three Unpaired Electrons
The presence of three unpaired electrons makes nitrogen a highly reactive element. These unpaired electrons readily participate in covalent bonding with other atoms, leading to the formation of stable molecules. Nitrogen readily forms three covalent bonds to achieve a stable octet (eight electrons) in its outer shell. This is clearly evident in molecules like ammonia (NH₃) and nitrogen trifluoride (NF₃). In these molecules, nitrogen shares its three unpaired electrons with hydrogen and fluorine atoms, respectively.
Examples of Nitrogen's Bonding Behavior
- Ammonia (NH₃): Nitrogen forms three covalent bonds with three hydrogen atoms, each hydrogen atom sharing one electron with nitrogen. This results in a stable ammonia molecule with nitrogen achieving an octet.
- Nitrogen Trifluoride (NF₃): Similar to ammonia, nitrogen shares its three unpaired electrons with three fluorine atoms, forming three covalent bonds and achieving a stable octet.
- Nitric Oxide (NO): While less straightforward, even in nitric oxide (NO), one unpaired electron exists on both nitrogen and oxygen, resulting in a strong bond.
Exception: Dinitrogen (N₂)
The diatomic nitrogen molecule (N₂) is a notable exception. In this molecule, each nitrogen atom shares three electrons with the other nitrogen atom through triple covalent bonds, achieving a stable octet for both nitrogen atoms. Although each atom starts with three unpaired electrons, these are all paired in the final N₂ molecule. This strong triple bond accounts for nitrogen's relative inertness at room temperature.
The Importance of Nitrogen in Biological Systems
The unique electronic configuration and reactivity of nitrogen are fundamental to its crucial role in biological systems. Nitrogen is a key component of amino acids, the building blocks of proteins. It is also a constituent of nucleic acids (DNA and RNA), which carry genetic information. The nitrogen cycle, involving the conversion of nitrogen between various forms, is essential for maintaining life on Earth.
Nitrogen Fixation
Nitrogen fixation, a process where atmospheric nitrogen (N₂) is converted into ammonia (NH₃), is a crucial step in the nitrogen cycle. This process, often carried out by specialized bacteria, involves breaking the strong triple bond in N₂ and incorporating the nitrogen into biologically available forms. This highlights the importance of understanding nitrogen's bonding characteristics and its reactivity.
Conclusion: The Unpaired Electrons Define Nitrogen's Chemistry
In conclusion, nitrogen possesses three unpaired electrons in its ground state electron configuration (1s²2s²2p³). These unpaired electrons are responsible for nitrogen's remarkable reactivity and its ability to form a wide variety of stable compounds. This fundamental aspect of nitrogen's atomic structure is crucial for understanding its chemical behavior, its importance in biological systems, and its indispensable role in various industrial processes. The presence of these unpaired electrons drives nitrogen's interactions, making it a vital element for life and countless applications in the world around us. From the strong triple bond in atmospheric nitrogen gas to the essential roles in amino acids and nucleic acids, the three unpaired electrons in a nitrogen atom's valence shell underpin its significance. The careful study of this seemingly simple element yields a wealth of understanding regarding the intricacies of atomic structure and chemical bonding.
Latest Posts
Latest Posts
-
Network Layer Firewall Works As A
Mar 31, 2025
-
Is Sodium Methoxide A Strong Nucleophile
Mar 31, 2025
-
The Amount Of Space An Object Occupies
Mar 31, 2025
-
A Small Object Begins A Free Fall From A Height
Mar 31, 2025
-
Which Strand Of Dna Serves As The Template For Transcription
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Unpaired Electrons Does Nitrogen Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
