How Many Stereoisomers Are Possible For 2 4-hexadiene
News Leon
Mar 25, 2025 · 4 min read
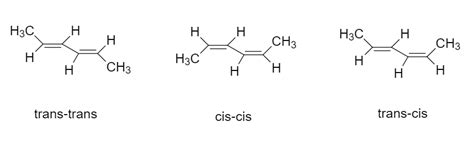
Table of Contents
How Many Stereoisomers are Possible for 2,4-Hexadiene? A Deep Dive into Isomerism
Determining the number of possible stereoisomers for a given molecule is a fundamental concept in organic chemistry. This article will delve into the detailed analysis of 2,4-hexadienes, exploring the types of isomerism present and meticulously calculating the total number of stereoisomers. We will unpack the concepts of conformational isomers, cis-trans isomerism (geometric isomerism), and optical isomerism (enantiomerism and diastereomerism) to arrive at a definitive answer.
Understanding Isomerism in Organic Molecules
Isomers are molecules with the same molecular formula but different structural formulas. This seemingly simple definition encompasses a wide variety of structural variations, leading to different chemical and physical properties. The primary types of isomerism relevant to 2,4-hexadiene are:
1. Constitutional Isomerism (Structural Isomerism)
Constitutional isomers possess the same molecular formula but differ in the connectivity of their atoms. This means that the atoms are bonded together in a different order. For 2,4-hexadiene, constitutional isomers are less common, as the specific arrangement of the double bonds significantly defines the structure. However, other constitutional isomers with the formula C₆H₁₀ are possible, though they are not 2,4-hexadienes.
2. Stereoisomerism
Stereoisomers share the same molecular formula and connectivity of atoms but differ in the spatial arrangement of their atoms. This is further divided into two key categories:
a) Geometric Isomerism (Cis-Trans Isomerism)
Geometric isomers, or cis-trans isomers, arise due to the restricted rotation around a double bond. The substituents on each carbon of the double bond can be arranged either on the same side (cis) or on opposite sides (trans). This is directly applicable to 2,4-hexadiene, which contains two double bonds, each capable of exhibiting cis-trans isomerism.
b) Optical Isomerism (Enantiomerism and Diastereomerism)
Optical isomers are stereoisomers that are non-superimposable mirror images of each other. These isomers rotate plane-polarized light in opposite directions. Enantiomers are a pair of non-superimposable mirror images, while diastereomers are stereoisomers that are not mirror images of each other.
The presence of chiral centers (carbon atoms bonded to four different groups) is a prerequisite for optical isomerism. 2,4-hexadiene does not possess chiral centers in its basic structure, but certain substituted derivatives might.
Analyzing 2,4-Hexadiene for Stereoisomers
Let's systematically analyze the possible stereoisomers of 2,4-hexadiene:
The molecule has two double bonds, located between carbons 2-3 and 4-5. Each double bond can exist in either a cis or trans configuration.
Therefore, we have the following possibilities:
- Double Bond 1 (C2-C3): Cis or Trans
- Double Bond 2 (C4-C5): Cis or Trans
This leads to a total of 2 x 2 = 4 possible geometric isomers. Let's represent these isomers:
-
(2Z,4Z)-2,4-Hexadiene: Both double bonds are in the cis configuration.
-
(2Z,4E)-2,4-Hexadiene: Double bond 1 is cis, double bond 2 is trans.
-
(2E,4Z)-2,4-Hexadiene: Double bond 1 is trans, double bond 2 is cis.
-
(2E,4E)-2,4-Hexadiene: Both double bonds are in the trans configuration.
The Z and E notation (formerly cis and trans) is used to unambiguously describe the configuration around each double bond. Z stands for "zusammen," meaning together, indicating that the higher priority groups are on the same side of the double bond. E stands for "entgegen," meaning opposite, indicating that the higher priority groups are on opposite sides of the double bond.
Important Note: The use of Z/E nomenclature is crucial for accurate representation, especially as molecule complexity increases. Simple cis/trans designations may become ambiguous.
Considering Conformational Isomers
While the four geometric isomers above represent the major stereoisomeric forms, 2,4-hexadiene also exhibits conformational isomerism. Conformational isomers (or conformers) are different spatial arrangements of a molecule that can interconvert by rotation around single bonds.
Due to the rotation around the single C3-C4 bond, each of the four geometric isomers exists in multiple conformations. These conformations differ in energy due to steric interactions (spatial crowding of atoms). However, these conformational isomers are rapidly interconverting at room temperature and are generally not considered distinct stereoisomers in the context of counting the number of stereoisomers.
Absence of Optical Isomers in the Un-substituted Molecule
As previously mentioned, 2,4-hexadiene, in its unsubstituted form (meaning no additional groups or atoms replacing hydrogens), lacks chiral centers. Therefore, it does not exhibit optical isomerism; it does not have enantiomers or diastereomers.
Conclusion: Total Number of Stereoisomers
Based on our analysis, the total number of stereoisomers for 2,4-hexadiene is four. These four stereoisomers are distinct geometric isomers resulting from the possible cis-trans configurations around the two double bonds. While conformational isomers exist due to rotation around the single bond, they are rapidly interconverting and are typically not counted as separate stereoisomers. The absence of chiral centers eliminates the possibility of optical isomerism.
Therefore, a comprehensive understanding of isomerism, including geometric isomerism and conformational isomerism, is crucial for accurately determining the number of possible stereoisomers for a given molecule. The use of systematic nomenclature, like the Z/E system, is vital for unambiguous representation and analysis of molecular structures. This detailed analysis provides a clear and complete understanding of the stereoisomerism in 2,4-hexadiene.
Latest Posts
Latest Posts
-
Oxidation Number Of Cr In K2cr2o7
Mar 28, 2025
-
Matter Is Anything That Has And Takes Up
Mar 28, 2025
-
Actinoid Contraction Is Greater Than Lanthanoid Contraction
Mar 28, 2025
-
Anything That Occupies Space And Has Mass Is Called
Mar 28, 2025
-
Which Of The Following Is Not Correct
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Stereoisomers Are Possible For 2 4-hexadiene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
