Anything That Occupies Space And Has Mass Is Called
News Leon
Mar 28, 2025 · 7 min read
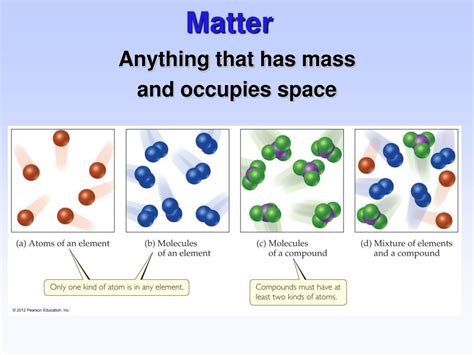
Table of Contents
Anything That Occupies Space and Has Mass Is Called Matter: A Deep Dive into the Building Blocks of Our Universe
The simple statement, "Anything that occupies space and has mass is called matter," forms the foundational bedrock of physics and chemistry. Understanding matter goes beyond a simple definition; it's a journey into the very fabric of our universe, exploring its diverse forms, properties, and interactions. This comprehensive article delves deep into the concept of matter, examining its various states, classifications, properties, and the fundamental particles that constitute it. We’ll also touch upon the relationship between matter and energy, a cornerstone of modern physics.
Defining Matter: More Than Just Space and Mass
While the initial definition—occupying space and possessing mass—is accurate, it's crucial to unpack its components. Space, in this context, refers to volume—the three-dimensional extent of an object. Think of a rock, a cloud, or even air; they all occupy a certain amount of space. Mass, on the other hand, represents the amount of matter contained within that space. Mass is often confused with weight, but they're distinct. Weight is the force of gravity acting on an object's mass; it changes depending on the gravitational field, while mass remains constant.
Therefore, matter encompasses everything with both volume and mass, from the smallest subatomic particles to the largest celestial bodies. This broad definition includes solids, liquids, gases, and plasma, as well as more exotic states of matter like Bose-Einstein condensates and fermionic condensates, which exist under extreme conditions.
The States of Matter: A Multifaceted Reality
Matter exists in various states, primarily determined by the arrangement and interaction of its constituent particles. These states aren't rigidly defined; transitions between them are common and often influenced by factors like temperature and pressure.
1. Solids: Structure and Stability
Solids are characterized by their fixed shape and volume. Their constituent particles (atoms, molecules, or ions) are tightly packed in a highly ordered arrangement, resulting in strong intermolecular forces. This structure gives solids their rigidity and resistance to changes in shape or volume. Examples of solids abound: rocks, ice, wood, metals, and crystals.
2. Liquids: Flow and Adaptability
Liquids maintain a constant volume but adopt the shape of their container. Their particles are closer together than in gases but have more freedom of movement than in solids, leading to fluidity. Intermolecular forces are weaker than in solids, allowing for flow and diffusion. Water, oil, and mercury are everyday examples of liquids.
3. Gases: Expansion and Compression
Gases lack both a fixed shape and volume, expanding to fill their container completely. Their particles are widely dispersed with weak intermolecular forces, resulting in high compressibility and expansibility. Air, oxygen, and carbon dioxide are examples of gases.
4. Plasma: Ionized Excitement
Plasma, often considered the fourth state of matter, is a highly energized state characterized by ionized particles—atoms that have lost or gained electrons, resulting in a mixture of free electrons and positively charged ions. Plasma exhibits unique properties due to its charged nature, making it highly conductive and responsive to electromagnetic fields. The sun and stars are primarily composed of plasma, and it's also found in fluorescent lights and lightning strikes.
5. Exotic States: Beyond the Familiar
Beyond the four primary states, there exist several exotic states of matter observable under extreme conditions:
- Bose-Einstein Condensates (BECs): At extremely low temperatures, some atoms lose their individual identities and behave as a single entity, forming a macroscopic quantum state.
- Fermionic Condensates: Similar to BECs but formed from fermions (particles that obey the Pauli exclusion principle), exhibiting unique properties.
- Quark-Gluon Plasma (QGP): This state exists at extremely high temperatures and densities, where quarks and gluons, the fundamental constituents of protons and neutrons, are no longer confined within nucleons.
Classifying Matter: Pure Substances and Mixtures
Matter can be further classified into pure substances and mixtures.
1. Pure Substances: Elements and Compounds
Pure substances have a uniform composition throughout and cannot be separated into simpler components by physical means. They are categorized into:
- Elements: The fundamental building blocks of matter, composed of atoms with the same number of protons. Examples include oxygen (O), hydrogen (H), and iron (Fe). The periodic table organizes and categorizes all known elements.
- Compounds: Substances formed by the chemical combination of two or more elements in fixed proportions. Water (H₂O), salt (NaCl), and carbon dioxide (CO₂) are examples of compounds.
2. Mixtures: Homogeneous and Heterogeneous
Mixtures are combinations of two or more substances that retain their individual properties and can be separated by physical means. They are categorized into:
- Homogeneous Mixtures: Have a uniform composition throughout, meaning the components are evenly distributed. Examples include saltwater and air.
- Heterogeneous Mixtures: Have a non-uniform composition, with visible distinct components. Examples include sand and water, or a salad.
Properties of Matter: Identifying and Characterizing
Matter exhibits various properties, which can be broadly categorized into physical and chemical properties.
1. Physical Properties: Observable Characteristics
Physical properties can be observed or measured without changing the chemical composition of the matter. These include:
- Density: Mass per unit volume.
- Melting Point: The temperature at which a solid changes to a liquid.
- Boiling Point: The temperature at which a liquid changes to a gas.
- Solubility: The ability of a substance to dissolve in another substance.
- Conductivity: The ability of a substance to conduct heat or electricity.
- Color, Odor, and Texture: Observable characteristics.
2. Chemical Properties: Reactivity and Transformation
Chemical properties describe how a substance reacts with other substances or changes its chemical composition. These properties are only observed during a chemical reaction:
- Flammability: The ability of a substance to burn.
- Reactivity with acids or bases: How a substance interacts with acids or bases.
- Oxidation: The reaction of a substance with oxygen.
- Decomposition: The breaking down of a substance into simpler components.
The Fundamental Building Blocks: Atoms and Subatomic Particles
At the heart of matter lies the atom, the smallest unit of an element that retains its chemical properties. Atoms are composed of even smaller particles:
- Protons: Positively charged particles found in the nucleus.
- Neutrons: Neutral particles found in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus.
The number of protons in an atom's nucleus determines its atomic number and identifies the element. Isotopes of an element have the same number of protons but differ in the number of neutrons.
Furthermore, protons and neutrons are themselves composed of even smaller particles called quarks, which are held together by gluons. These fundamental particles, along with electrons and other leptons, form the Standard Model of particle physics, a comprehensive theory describing the fundamental constituents of matter and their interactions.
Matter and Energy: An Interchangeable Relationship
Einstein's famous equation, E=mc², reveals a profound relationship between matter and energy. This equation demonstrates that mass (m) and energy (E) are equivalent and interchangeable, with the speed of light (c) as the conversion factor. Nuclear reactions, such as fission and fusion, demonstrate this equivalence, where a small amount of mass is converted into a large amount of energy. This principle underpins the power of nuclear weapons and nuclear power plants.
Conclusion: A Universe Built on Matter
From the smallest quark to the largest galaxy, everything in the observable universe is composed of matter. Understanding the nature of matter, its states, classifications, properties, and fundamental constituents is crucial to comprehending the universe's workings. This exploration goes beyond a simple definition; it unveils a fascinating world of intricate structures, interactions, and transformations that continue to be a subject of intense scientific inquiry and discovery. Further research continues to refine our understanding of matter, pushing the boundaries of knowledge and revealing new insights into the fundamental building blocks of our reality. The journey of discovery continues, promising even more profound revelations about the nature of matter in the years to come.
Latest Posts
Latest Posts
-
Weather Averaged Over A Long Period Of Time
Mar 31, 2025
-
Receptors For Nonsteroid Hormones Are Located In
Mar 31, 2025
-
What Is The Measure Of 3
Mar 31, 2025
-
Unlike A Eukaryotic Cell A Prokaryotic Cell Does Not Have
Mar 31, 2025
-
Find The Value Of In The Triangle Shown Below
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Anything That Occupies Space And Has Mass Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
