How Many Single Covalent Bonds Can Carbon Form
News Leon
Mar 18, 2025 · 5 min read
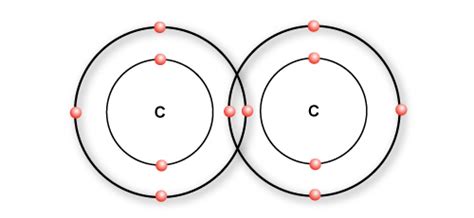
Table of Contents
How Many Single Covalent Bonds Can Carbon Form? Understanding Carbon's Bonding Capacity
Carbon, the backbone of life, holds a unique position in the periodic table. Its ability to form a specific number of covalent bonds is fundamental to its unparalleled versatility and the vast diversity of organic molecules found in nature and synthesized in laboratories. This article delves deep into the question: How many single covalent bonds can carbon form? We'll explore the underlying reasons, the implications of this bonding capacity, and its profound impact on the complexity of organic chemistry.
Understanding Covalent Bonding
Before we delve into carbon's bonding prowess, let's briefly review the concept of covalent bonding. Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration, typically a full outer electron shell. This sharing of electrons results in a strong attractive force holding the atoms together. The number of covalent bonds an atom can form is primarily determined by the number of unpaired electrons it possesses in its outermost shell, also known as its valence shell.
Carbon's Electronic Configuration: The Key to its Bonding
Carbon, with its atomic number of 6, has an electronic configuration of 1s²2s²2p². This means it has two electrons in the first energy level (1s²) and four electrons in the second energy level (2s²2p²). Crucially, it has four electrons in its valence shell (2s²2p²). These four electrons are available for bonding.
The Excitation of Electrons: Enabling Four Bonds
While the ground state configuration suggests only two unpaired electrons (in the 2p orbitals), carbon can readily promote one electron from the 2s orbital to an empty 2p orbital. This process, known as electron excitation, results in four unpaired electrons – one in the 2s orbital and three in the 2p orbitals. Each of these unpaired electrons can participate in a covalent bond, explaining carbon's ability to form four single covalent bonds.
The Tetrahedral Geometry: A Consequence of Four Bonds
The formation of four single covalent bonds by carbon doesn't happen randomly. The four bonds arrange themselves spatially in a tetrahedral geometry. This means the four bonds point towards the corners of a tetrahedron, a three-dimensional shape with four equilateral triangular faces. This specific arrangement minimizes electron-electron repulsion and maximizes stability. The bond angle in a tetrahedral structure is approximately 109.5 degrees.
Examples of Carbon's Four Single Covalent Bonds:
Carbon's ability to form four single covalent bonds is the foundation of organic chemistry. Let's look at some quintessential examples:
Methane (CH₄):
Methane is the simplest hydrocarbon, consisting of a central carbon atom bonded to four hydrogen atoms. Each hydrogen atom contributes one electron to form a single covalent bond with the carbon atom. This results in a stable, tetrahedral molecule.
Ethane (C₂H₆):
Ethane showcases a carbon-carbon single bond. Two carbon atoms are connected by a single covalent bond, and each carbon atom then forms three additional single bonds with hydrogen atoms. The overall molecule adopts a zig-zag shape resulting from the tetrahedral geometry around each carbon.
Ethanol (C₂H₅OH):
Ethanol, a common alcohol, exemplifies carbon's versatility in bonding with different atoms. The two carbon atoms are linked by a single bond. One carbon atom bonds to three hydrogen atoms and one oxygen atom (through a single bond). The oxygen atom, in turn, bonds to a hydrogen atom, forming the hydroxyl (-OH) group.
Carbon's Capacity for Multiple Bonds: Double and Triple Bonds
While carbon predominantly forms four single covalent bonds, it's also capable of forming double and triple bonds. These multiple bonds involve the sharing of two or three electron pairs between two atoms, respectively. However, the total number of bonds (single bond equivalents) remains four.
Ethene (C₂H₄):
Ethene (ethylene) exhibits a carbon-carbon double bond. Each carbon atom forms two single bonds with hydrogen atoms and one double bond with the other carbon atom. The double bond consists of one sigma bond and one pi bond.
Ethyne (C₂H₂):
Ethyne (acetylene) displays a carbon-carbon triple bond. Each carbon atom forms one single bond with a hydrogen atom and one triple bond with the other carbon atom. This triple bond consists of one sigma bond and two pi bonds.
It's crucial to remember that despite forming double or triple bonds, the total number of electron pairs shared by each carbon atom is still four. The multiple bonds only represent different ways of achieving this bonding capacity.
Implications of Carbon's Bonding Capacity
Carbon's remarkable ability to form four covalent bonds has far-reaching consequences:
- Vast structural diversity: This capacity allows carbon to form long chains, branched chains, and ring structures, leading to an astronomical number of possible organic molecules.
- Isomerism: Molecules with the same molecular formula but different structural arrangements (isomers) are commonplace due to carbon's ability to form various branched chains and ring structures.
- Functional groups: The diverse ways carbon can bond with other atoms (oxygen, nitrogen, sulfur, halogens, etc.) give rise to a wide range of functional groups that determine the chemical properties of organic molecules.
- Chirality: Carbon's tetrahedral geometry can lead to chiral molecules, which exhibit handedness. This characteristic has significant implications in biochemistry and pharmacology.
Beyond Organic Chemistry: Carbon's Role in Inorganic Compounds
While carbon's tetravalent nature is most prominently seen in organic chemistry, it also forms four bonds in various inorganic compounds. Examples include carbon tetrachloride (CCl₄), where carbon is bonded to four chlorine atoms, and silicon carbide (SiC), a covalent network solid where carbon atoms are bonded tetrahedrally to silicon atoms.
Conclusion: Carbon's Unique Position
The answer to "How many single covalent bonds can carbon form?" is definitively four. This seemingly simple fact underpins the immense complexity and diversity of organic molecules, impacting fields from medicine and materials science to environmental chemistry and astrobiology. Understanding carbon's bonding capacity is fundamental to grasping the building blocks of life and the myriad ways in which carbon atoms interact to create the incredible array of substances found in our world. Further exploration into carbon's bonding behavior, including the nuances of hybrid orbitals and the influence of molecular geometry, opens up even deeper understanding of this crucial element.
Latest Posts
Latest Posts
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Single Covalent Bonds Can Carbon Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
