How Many Orbitals In P Subshell
News Leon
Mar 29, 2025 · 6 min read
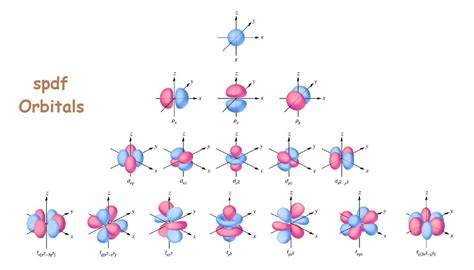
Table of Contents
How Many Orbitals in a P Subshell? A Deep Dive into Atomic Structure
Understanding the arrangement of electrons within an atom is fundamental to chemistry. This article delves into the intricacies of atomic orbitals, focusing specifically on the p subshell and answering the crucial question: how many orbitals are present within it? We'll explore the quantum numbers that define these orbitals, their shapes, and their significance in determining an atom's properties and reactivity.
Understanding Atomic Orbitals and Quantum Numbers
Before diving into the p subshell, let's establish a foundation in atomic structure. Electrons don't simply orbit the nucleus in random paths; they occupy specific regions of space called atomic orbitals. These orbitals are described by four quantum numbers:
-
Principal Quantum Number (n): This number defines the energy level of the electron and the size of the orbital. It can take on positive integer values (n = 1, 2, 3, ...). Higher 'n' values indicate higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number defines the shape of the orbital and the subshell it belongs to. It can take on integer values from 0 to n-1. For example, if n = 2, l can be 0 or 1. These values correspond to specific subshells: l = 0 (s subshell), l = 1 (p subshell), l = 2 (d subshell), l = 3 (f subshell), and so on.
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can take on integer values from -l to +l, including 0. This means that for a given subshell, there are 2l + 1 orbitals with different orientations.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can only have two values: +1/2 (spin up) or -1/2 (spin down). Each orbital can hold a maximum of two electrons, one with spin up and one with spin down (Pauli Exclusion Principle).
The P Subshell: Shape and Orientation
Now let's focus on the p subshell, characterized by an azimuthal quantum number (l) of 1. This means that the magnetic quantum number (ml) can have three possible values: -1, 0, and +1. Therefore, there are three p orbitals in a p subshell.
These three p orbitals have distinct spatial orientations, commonly designated as px, py, and pz. Unlike the spherical s orbitals, p orbitals have a dumbbell shape, with two lobes of electron density on either side of the nucleus.
-
px orbital: This orbital has its electron density concentrated along the x-axis.
-
py orbital: This orbital has its electron density concentrated along the y-axis.
-
pz orbital: This orbital has its electron density concentrated along the z-axis.
It's crucial to remember that these are just representations; the actual electron distribution is more complex and probabilistic. The orbitals are regions of space where the probability of finding an electron is high.
Visualizing the P Orbitals
Imagine three mutually perpendicular axes (x, y, z) intersecting at the nucleus. The px orbital extends along the x-axis, the py along the y-axis, and the pz along the z-axis. Each orbital has a nodal plane—a plane where the probability of finding an electron is zero—passing through the nucleus. The px orbital has a nodal plane containing the y and z axes; the py orbital has a nodal plane containing the x and z axes; and the pz orbital has a nodal plane containing the x and y axes.
Significance of the Three P Orbitals
The presence of three p orbitals has significant consequences for the chemical behavior of elements. The ability of atoms to form bonds depends on the availability of unpaired electrons in their valence orbitals. Since the p subshell can accommodate up to six electrons (two in each of the three orbitals), atoms with valence electrons in the p subshell exhibit a wide range of bonding possibilities, leading to diverse molecular structures and properties.
Filling the P Subshell: Hund's Rule and Electron Configuration
When filling the p subshell with electrons, Hund's rule comes into play. Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is because electrons, being negatively charged, repel each other and prefer to occupy separate orbitals with parallel spins (maximizing their spin multiplicity) to minimize electron-electron repulsion.
For example, consider nitrogen (N), which has seven electrons. Its electron configuration is 1s²2s²2p³. The three 2p electrons will occupy the three 2p orbitals individually, each with parallel spin, before any pairing occurs.
Beyond the Basics: Hybridization and Molecular Orbital Theory
The simple picture of atomic orbitals presented above forms the basis for understanding more complex concepts like hybridization and molecular orbital theory. Hybridization explains how atomic orbitals can combine to form hybrid orbitals with different shapes and energies, influencing molecular geometry and bonding properties. Molecular orbital theory describes how atomic orbitals combine to form molecular orbitals, providing a more accurate representation of bonding in molecules.
Applications and Importance in Chemistry
The understanding of p orbitals is fundamental to various areas of chemistry:
-
Organic Chemistry: The geometry and reactivity of organic molecules are largely determined by the hybridization of carbon atoms' sp³, sp², and sp orbitals (which involve p orbitals).
-
Inorganic Chemistry: The coordination chemistry of transition metals heavily relies on the availability and interactions of d and p orbitals.
-
Spectroscopy: The transitions of electrons between different orbitals, including p orbitals, are responsible for the absorption and emission of light, forming the basis of various spectroscopic techniques.
-
Materials Science: The properties of materials, including semiconductors and conductors, are directly related to the electronic structure and the arrangement of electrons in their constituent atoms, including the p orbitals.
Advanced Concepts: Relativistic Effects and Quantum Mechanics
While the simple picture of p orbitals provides a good starting point, more advanced quantum mechanical treatments reveal complexities. Relativistic effects, particularly significant for heavier elements, can alter the energies and shapes of atomic orbitals, including p orbitals.
Conclusion: The Crucial Role of the P Subshell
In summary, the p subshell is a key component of atomic structure, containing three p orbitals with specific shapes and orientations. The presence of these three orbitals is crucial for understanding the diverse chemical behavior of elements, molecular geometry, bonding, and numerous spectroscopic phenomena. This deep dive into the p subshell highlights the fundamental importance of understanding atomic orbitals in chemistry and related scientific disciplines. From simple electron configurations to complex molecular orbital theories, the three p orbitals remain a cornerstone of our comprehension of the atomic world.
Latest Posts
Latest Posts
-
28 9 As A Mixed Number
Apr 01, 2025
-
What Are The Advantages Of Sexual Reproduction Over Asexual Reproduction
Apr 01, 2025
-
Is A Base A Proton Acceptor
Apr 01, 2025
-
Why Is Africa Called A Plateau Continent
Apr 01, 2025
-
A Carbohydrate That Makes Up The Cell Walls Of Plants
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Orbitals In P Subshell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
