How Many O2 Molecules Can Hemoglobin Carry
News Leon
Mar 25, 2025 · 6 min read
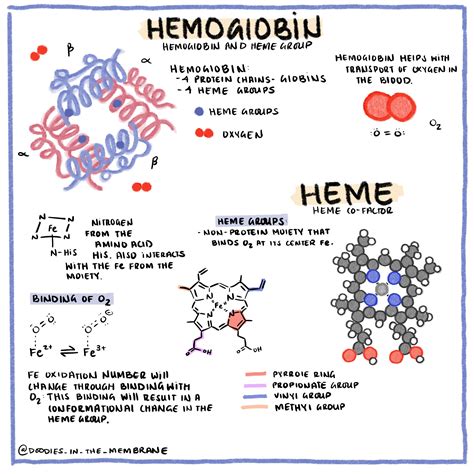
Table of Contents
How Many O2 Molecules Can Hemoglobin Carry? Understanding Oxygen Transport in the Blood
Oxygen is essential for life. Our bodies rely on a constant supply to fuel cellular respiration, the process that generates the energy needed for all bodily functions. But how does oxygen, a gas, travel from our lungs to the furthest reaches of our tissues? The answer lies in a remarkable protein found in our red blood cells: hemoglobin. This article delves deep into the fascinating world of hemoglobin, exploring its structure, function, and the precise number of oxygen molecules it can transport.
The Marvel of Hemoglobin: Structure and Function
Hemoglobin is a tetrameric protein, meaning it's composed of four subunits. Each subunit is remarkably similar in structure and contains a heme group. This is where the magic happens.
The Heme Group: The Oxygen Binding Site
The heme group is a porphyrin ring complex containing a single iron (Fe) ion at its center. This iron ion is crucial for oxygen binding. In its ferrous state (Fe²⁺), the iron atom can reversibly bind to one oxygen molecule (O₂). This reversible binding is absolutely key to hemoglobin's ability to both pick up oxygen in the lungs and release it in the tissues.
Hemoglobin Subunits: Alpha and Beta Chains
Human adult hemoglobin (HbA) consists of two alpha (α) and two beta (β) globin chains. Each chain is a polypeptide chain, folded into a specific three-dimensional structure. These chains are not identical, however, they work in concert for efficient oxygen transport. The precise arrangement of these chains influences hemoglobin's affinity for oxygen.
Oxygen Binding and the Cooperative Effect
The most fascinating aspect of hemoglobin's function is its cooperative binding of oxygen. This means that the binding of one oxygen molecule to a heme group influences the binding of subsequent oxygen molecules to other heme groups within the same hemoglobin molecule.
The Sigmoid Oxygen-Hemoglobin Dissociation Curve
The cooperative binding is reflected in the sigmoid (S-shaped) oxygen-hemoglobin dissociation curve. This curve illustrates the relationship between the partial pressure of oxygen (pO₂) and the percentage of hemoglobin saturated with oxygen. The sigmoid shape is a direct consequence of the cooperative binding:
- At low pO₂: Hemoglobin has a low affinity for oxygen. The binding of the first oxygen molecule is relatively difficult.
- As pO₂ increases: The binding of subsequent oxygen molecules becomes progressively easier due to the conformational changes induced by the initial binding. This leads to a rapid increase in oxygen saturation.
- At high pO₂: Hemoglobin becomes almost fully saturated with oxygen.
Allosteric Regulation: Factors Influencing Oxygen Binding
The affinity of hemoglobin for oxygen is not constant. Several factors can influence its oxygen-binding capacity, affecting the position of the oxygen-hemoglobin dissociation curve:
- pH (Bohr Effect): A decrease in pH (increased acidity) reduces hemoglobin's affinity for oxygen, promoting oxygen release in metabolically active tissues where CO₂ production is high.
- Carbon Dioxide (CO₂): CO₂ directly binds to hemoglobin, reducing its oxygen affinity. The presence of CO₂ also contributes to the Bohr effect.
- 2,3-Bisphosphoglycerate (2,3-BPG): This molecule binds to hemoglobin, reducing its oxygen affinity. 2,3-BPG levels increase during situations of low oxygen availability, such as high altitude, facilitating oxygen release in the tissues.
- Temperature: Increased temperature decreases hemoglobin's affinity for oxygen.
Calculating the Number of O2 Molecules: A Step-by-Step Approach
Now, let's get to the core question: how many oxygen molecules can one hemoglobin molecule carry?
1. Heme Groups per Hemoglobin Molecule: Since hemoglobin has four subunits, and each subunit contains one heme group, there are a total of four heme groups per hemoglobin molecule.
2. Oxygen Molecules per Heme Group: Each heme group can bind to one oxygen molecule.
3. Total Oxygen Molecules: Therefore, one hemoglobin molecule can carry a maximum of four oxygen molecules.
Important Note: This is the theoretical maximum. In reality, the percentage of hemoglobin molecules carrying the maximum number of oxygen molecules depends on the pO₂ and the factors influencing oxygen binding mentioned earlier.
Beyond the Basics: Hemoglobin Variants and Clinical Significance
While HbA is the most common form of hemoglobin, several variants exist. Some are harmless polymorphisms, while others can cause significant health problems. Examples include:
- Hemoglobin S (HbS): The causative agent of sickle cell anemia, resulting from a single amino acid substitution in the beta-globin chain. This leads to abnormal hemoglobin polymerization and red blood cell sickling.
- Hemoglobin C (HbC): Another variant caused by a single amino acid substitution, leading to mild hemolytic anemia.
- Thalassemia: A group of inherited blood disorders characterized by reduced or absent production of globin chains.
Understanding the structure and function of hemoglobin, including its capacity to bind four oxygen molecules, is crucial for comprehending oxygen transport, gas exchange, and the pathophysiology of various blood disorders. Research continues to unravel the complexities of this vital protein, leading to improved diagnostic tools and therapeutic strategies for hemoglobin-related diseases.
The Impact of Altitude and Exercise on Hemoglobin Function
The body adapts to various conditions by altering hemoglobin function. This is especially true at high altitudes and during strenuous exercise.
High Altitude Adaptation
At high altitudes, the partial pressure of oxygen is significantly lower. This triggers several physiological adaptations, including:
- Increased 2,3-BPG levels: This facilitates the release of oxygen from hemoglobin in the tissues, even at low pO₂.
- Increased erythropoietin production: This hormone stimulates the bone marrow to produce more red blood cells, increasing the overall oxygen-carrying capacity of the blood.
- Increased lung ventilation: The body increases breathing rate and depth to compensate for the lower oxygen availability.
Exercise and Oxygen Demand
During intense exercise, oxygen demand increases dramatically. The body responds by:
- Increased cardiac output: The heart pumps more blood per minute, delivering more oxygen to the muscles.
- Increased blood flow to muscles: Blood is shunted away from less essential organs to deliver more oxygen to the working muscles.
- Increased Bohr effect: The increased acidity in working muscles (due to lactic acid production) promotes oxygen release from hemoglobin.
These adaptations ensure that sufficient oxygen is delivered to meet the increased demands of exercise.
Conclusion: Hemoglobin's Crucial Role in Life
Hemoglobin's ability to bind and transport four oxygen molecules per molecule is a testament to the intricate design of biological systems. This remarkable protein is essential for life, ensuring that every cell receives the oxygen it needs to function. Further research into hemoglobin's structure, function, and regulation continues to reveal its complexities and potential for therapeutic interventions. Its role in oxygen transport is fundamental to understanding human physiology and pathology, emphasizing the importance of this crucial protein in maintaining our health and well-being.
Latest Posts
Latest Posts
-
1 Meter Equals How Many Millimeters
Mar 28, 2025
-
In The System Of Mass Production Unskilled Workers
Mar 28, 2025
-
What Is The Ph Of The Neutral Solution
Mar 28, 2025
-
Respiratory Control Centers Are Located In The
Mar 28, 2025
-
How Many Cm Is 25 Mm
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many O2 Molecules Can Hemoglobin Carry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
