How Many Neutrons Are In Mg
News Leon
Mar 15, 2025 · 5 min read
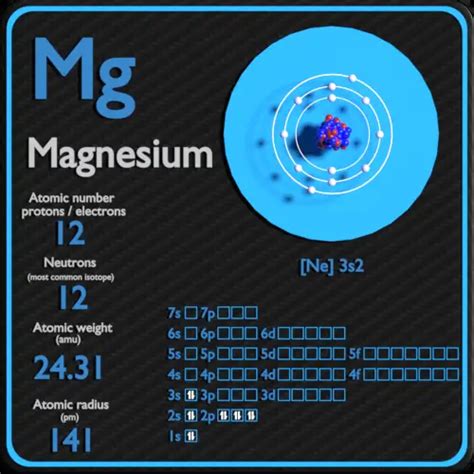
Table of Contents
How Many Neutrons Are in Mg? Isotopes and Atomic Structure
Magnesium (Mg), a vital element for life, presents a fascinating case study in atomic structure, particularly concerning the number of neutrons it possesses. Unlike the fixed number of protons defining an element (12 for Magnesium), the neutron count varies, leading to the concept of isotopes. Understanding this variation is crucial for comprehending magnesium's properties and its applications in various fields. This article will delve deep into the number of neutrons in magnesium, exploring its isotopes, their abundance, and the implications of this variability.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before exploring the neutron count in magnesium, let's revisit the fundamental components of an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; all magnesium atoms have 12 protons.
- Neutrons: Neutrally charged particles also residing in the nucleus. Unlike protons, the number of neutrons can vary within the same element, giving rise to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons usually equals the number of protons in a neutral atom.
The atomic number of an element is the number of protons in its nucleus (12 for magnesium). The mass number represents the total number of protons and neutrons in the nucleus. Because the number of neutrons can vary, the mass number is not a constant for a given element.
Magnesium Isotopes: A Spectrum of Neutron Numbers
Magnesium exists in nature as a mixture of three stable isotopes:
- Magnesium-24 (²⁴Mg): This is the most abundant isotope, comprising approximately 79% of naturally occurring magnesium. It has 12 protons and 12 neutrons (24 - 12 = 12 neutrons).
- Magnesium-25 (²⁵Mg): This isotope accounts for around 10% of natural magnesium. It has 12 protons and 13 neutrons (25 - 12 = 13 neutrons).
- Magnesium-26 (²⁶Mg): The least abundant stable isotope, making up about 11% of natural magnesium. It contains 12 protons and 14 neutrons (26 - 12 = 14 neutrons).
The notation "²⁴Mg," for example, indicates Magnesium with a mass number of 24. The superscript represents the mass number (protons + neutrons), while the subscript (often omitted) represents the atomic number (number of protons).
Why Isotopes Exist: Nuclear Stability
The existence of isotopes is linked to the forces within the atomic nucleus. The strong nuclear force binds protons and neutrons together, but the electrostatic repulsion between positively charged protons can destabilize larger nuclei. The neutron-to-proton ratio plays a critical role in nuclear stability. Different neutron numbers in magnesium isotopes result from variations in this ratio that still lead to stable nuclei.
Unstable Magnesium Isotopes: Radioactivity
While the three isotopes mentioned above are stable, several radioactive magnesium isotopes have been synthesized in laboratories. These unstable isotopes undergo radioactive decay, transforming into other elements over time. These isotopes have different neutron numbers than the stable isotopes and are characterized by their half-lives (the time it takes for half of a sample to decay).
Calculating the Average Number of Neutrons in Natural Magnesium
Since natural magnesium is a mixture of isotopes, we can calculate the average number of neutrons by considering the isotopic abundance of each isotope:
-
Calculate the weighted average of neutrons for each isotope:
- ²⁴Mg: 12 neutrons * 0.79 = 9.48 neutrons
- ²⁵Mg: 13 neutrons * 0.10 = 1.3 neutrons
- ²⁶Mg: 14 neutrons * 0.11 = 1.54 neutrons
-
Sum the weighted averages: 9.48 + 1.3 + 1.54 = 12.32 neutrons
Therefore, the average number of neutrons in a naturally occurring magnesium atom is approximately 12.32. It's crucial to remember that this is an average; no single magnesium atom in nature will possess exactly 12.32 neutrons.
Significance of Magnesium Isotopes: Applications and Research
The different isotopes of magnesium have varying applications and significance in different fields:
-
Geochemistry and Geology: The isotopic ratios of magnesium in rocks and minerals are valuable tools for geologists to understand geological processes, such as the formation of rocks, dating of geological events, and tracing the evolution of the Earth's mantle. Variations in isotopic ratios can indicate different geological sources or processes.
-
Biology and Medicine: Magnesium is essential for various biological processes, including enzyme activity, muscle contraction, and nerve transmission. Isotopic labeling techniques using stable magnesium isotopes (e.g., ²⁶Mg) are used in biomedical research to study magnesium metabolism, uptake, and transport in living organisms.
-
Materials Science: The properties of magnesium alloys can be subtly altered by controlling the isotopic composition. This opens possibilities for tailored material properties in specific applications.
-
Nuclear Physics: The study of radioactive magnesium isotopes helps researchers understand nuclear processes, decay mechanisms, and nuclear structure. These isotopes serve as valuable tools in nuclear research.
Beyond Magnesium: Isotopes and Their Importance
The concept of isotopes is not unique to magnesium; most elements exist as mixtures of isotopes. Isotopic analysis is a powerful tool across various scientific disciplines, including:
- Environmental Science: Tracing pollutants and studying environmental processes.
- Archaeology: Dating ancient artifacts and materials.
- Forensic Science: Analyzing evidence and identifying sources.
The variations in neutron numbers within isotopes of an element highlight the complexity and richness of atomic structure and its implications for the properties and behavior of matter. Understanding isotopes is crucial for advancements across a wide spectrum of scientific and technological fields.
Conclusion: The Dynamic Nature of Magnesium's Neutron Count
The number of neutrons in magnesium is not a fixed value but varies depending on the specific isotope. While the most common isotope, ²⁴Mg, possesses 12 neutrons, the presence of ²⁵Mg (13 neutrons) and ²⁶Mg (14 neutrons) in natural magnesium creates a spectrum of neutron counts. The average number of neutrons in naturally occurring magnesium is approximately 12.32. This seemingly simple detail reveals the intricate workings of atomic structure and underscores the importance of isotopes in diverse fields, from geology and biology to materials science and nuclear physics. Understanding this variability is crucial to comprehending magnesium's behavior and its significant role in the world around us. The study of magnesium isotopes serves as a microcosm of the broader significance of isotopic analysis in furthering our knowledge across scientific disciplines.
Latest Posts
Latest Posts
-
16 Out Of 40 As A Percentage
Mar 15, 2025
-
Which Of The Following Is A True Solution
Mar 15, 2025
-
How Many Vertices Does A Rectangular Pyramid Have
Mar 15, 2025
-
Which Are Different Forms Of The Same Gene
Mar 15, 2025
-
Which Of The Following Is A Population
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Are In Mg . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
