How Many Moles In 25 Grams Of Water
News Leon
Mar 25, 2025 · 5 min read
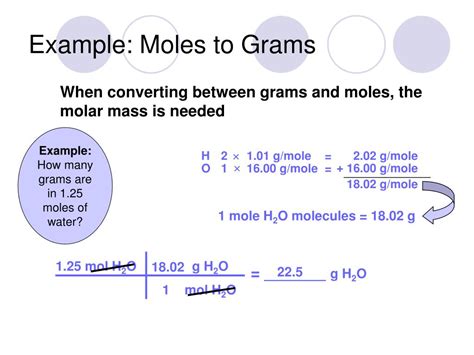
Table of Contents
How Many Moles Are in 25 Grams of Water? A Deep Dive into Moles and Molar Mass
Understanding moles is fundamental in chemistry. It's the bridge between the macroscopic world we see and the microscopic world of atoms and molecules. This article will comprehensively explain how to calculate the number of moles in 25 grams of water, delving into the concepts of molar mass, Avogadro's number, and practical applications.
Understanding Moles: The Chemist's Counting Unit
A mole (mol) is a unit of measurement in chemistry that represents Avogadro's number of particles. Avogadro's number is approximately 6.022 x 10<sup>23</sup>. This incredibly large number represents the number of atoms in 12 grams of carbon-12. Think of it as a chemist's way of counting incredibly large quantities of atoms or molecules, much like we use a dozen to represent 12 items.
Instead of dealing with individual atoms or molecules, which are far too small to count directly, chemists use moles to represent a specific number of these particles. This simplifies calculations and comparisons in chemical reactions and stoichiometry.
The Importance of Molar Mass
The molar mass of a substance is the mass of one mole of that substance. It's expressed in grams per mole (g/mol). To find the molar mass, you need to refer to the periodic table and sum the atomic masses of all the atoms in the molecule.
Calculating the Number of Moles in 25 Grams of Water (H₂O)
Water (H₂O) is a perfect example to illustrate the mole concept. Let's break down the calculation step-by-step to determine how many moles are in 25 grams of water:
Step 1: Determine the Molar Mass of Water
- Hydrogen (H): Atomic mass ≈ 1.008 g/mol
- Oxygen (O): Atomic mass ≈ 16.00 g/mol
Since water has two hydrogen atoms and one oxygen atom, its molar mass is:
(2 × 1.008 g/mol) + (1 × 16.00 g/mol) = 18.016 g/mol
Therefore, one mole of water weighs approximately 18.016 grams.
Step 2: Apply the Mole Formula
The formula to calculate the number of moles (n) is:
n = mass (m) / molar mass (M)
Where:
- n = number of moles
- m = mass of the substance (in grams)
- M = molar mass of the substance (in g/mol)
Step 3: Substitute the Values
In our case:
- m = 25 grams
- M = 18.016 g/mol
Substituting these values into the formula:
n = 25 g / 18.016 g/mol
n ≈ 1.388 moles
Therefore, there are approximately 1.388 moles in 25 grams of water.
Understanding the Result and its Implications
The calculation reveals that 25 grams of water contains approximately 1.388 moles. This means that there are approximately 1.388 x (6.022 x 10<sup>23</sup>) water molecules present in the 25-gram sample. This number is astronomically large, highlighting the immense number of molecules present even in a small amount of substance.
Practical Applications of Mole Calculations
The ability to calculate the number of moles is crucial in various chemical applications, including:
1. Stoichiometry: Balancing Chemical Equations
Stoichiometry uses mole ratios to determine the amounts of reactants and products involved in chemical reactions. Knowing the number of moles of a reactant allows you to calculate the amount of product formed or the amount of another reactant required.
Example: Consider the reaction of hydrogen and oxygen to form water:
2H₂ + O₂ → 2H₂O
If you have 1 mole of hydrogen gas (H₂), you can use the mole ratio (2:2) from the balanced equation to determine that you will produce 1 mole of water.
2. Concentration Calculations: Molarity
Molarity (M) is a common unit of concentration, defined as the number of moles of solute per liter of solution. Calculating moles is essential for preparing solutions of a specific concentration.
Example: To prepare 1 liter of a 1 M solution of sodium chloride (NaCl), you need to dissolve 1 mole of NaCl (its molar mass) in 1 liter of water.
3. Titrations: Determining Unknown Concentrations
Titrations are laboratory techniques used to determine the concentration of an unknown solution using a solution of known concentration. Mole calculations are crucial in interpreting the results of titrations.
4. Gas Laws: Relating Moles to Volume and Pressure
The Ideal Gas Law (PV = nRT) relates the pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). Calculating moles allows you to predict the volume of a gas at a specific pressure and temperature or vice-versa.
5. Mass Spectrometry: Identifying Compounds
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of ions. The resulting data, often expressed in terms of mass, needs mole calculations to determine the composition and molecular formula of the substance being analyzed.
Beyond the Basics: Advanced Concepts
While calculating moles in a simple substance like water is relatively straightforward, more complex scenarios may arise:
-
Hydrates: Hydrates are compounds that contain water molecules within their crystal structure. Calculating the moles of both the anhydrous compound and water in a hydrate sample requires considering the molar mass of both components.
-
Mixtures: Determining the moles of individual components in a mixture requires knowing the mass percentage or mole fraction of each component.
-
Reactions with Limiting Reactants: In chemical reactions involving multiple reactants, one reactant is often the limiting reactant. Mole calculations are essential in identifying the limiting reactant and calculating the theoretical yield of the product.
Conclusion: Moles – A Cornerstone of Chemistry
The ability to calculate the number of moles is paramount in various chemical applications. The concept of moles, coupled with molar mass and Avogadro's number, provides a practical way to handle the vast quantities of atoms and molecules involved in chemical reactions and processes. Understanding this fundamental concept allows for accurate stoichiometric calculations, precise solution preparation, and a deeper grasp of chemical interactions. While the calculation for 25 grams of water is a straightforward example, mastering this foundational knowledge opens doors to tackling more complex chemical problems and furthering your understanding of the chemical world. Remember, precise calculations depend on accurate measurements and a thorough understanding of the underlying chemical principles.
Latest Posts
Latest Posts
-
A Convex Lens Of Focal Length 10cm
Mar 26, 2025
-
Gases Have Indefinite Shape And Volume
Mar 26, 2025
-
Round 64 To The Nearest Ten
Mar 26, 2025
-
Which Of The Following Are Meso Compounds
Mar 26, 2025
-
Words Beginning With The Same Letter
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Moles In 25 Grams Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
