How Many Electrons Can The S Orbital Hold
News Leon
Mar 17, 2025 · 6 min read
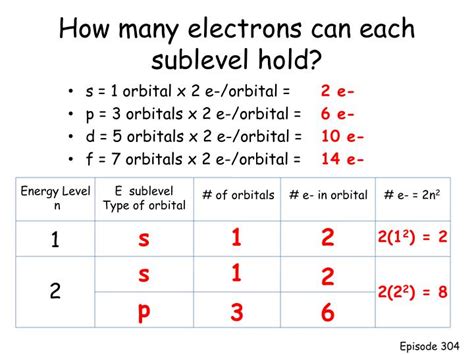
Table of Contents
How Many Electrons Can the s Orbital Hold? A Deep Dive into Atomic Structure
Understanding atomic structure is fundamental to grasping the behavior of matter. At the heart of this lies the concept of orbitals, regions of space within an atom where there's a high probability of finding an electron. One of the most basic, yet crucial, orbitals is the s orbital. But how many electrons can this seemingly simple orbital actually hold? The answer, and the journey to understanding it, unveils fascinating insights into the quantum world.
The s Orbital: A Spherical Haven for Electrons
The s orbital is the first and most fundamental type of atomic orbital. Its defining characteristic is its spherical shape. This doesn't mean electrons are confined to a solid sphere; rather, the probability of finding an electron decreases as you move further from the nucleus, creating a spherical distribution.
Principal Quantum Number (n) and Orbital Size
The size and energy level of an s orbital are determined by the principal quantum number (n). This quantum number can only take positive integer values (1, 2, 3, and so on). A higher n value indicates a larger orbital and higher energy level. For example, a 1s orbital is smaller and lower in energy than a 2s orbital.
Angular Momentum Quantum Number (l) and Orbital Shape
The shape of an orbital is described by the angular momentum quantum number (l). For s orbitals, l is always 0. This value of 0 uniquely defines the spherical symmetry of the s orbital. Other orbitals, such as p, d, and f, have different l values and consequently different shapes.
The Pauli Exclusion Principle: A Limit on Electron Occupancy
The key to determining how many electrons an s orbital can hold lies in the Pauli Exclusion Principle. This fundamental principle of quantum mechanics states that no two electrons in an atom can have the same set of four quantum numbers.
The Four Quantum Numbers: A Unique Electron Identity
Each electron in an atom is uniquely identified by a set of four quantum numbers:
- Principal Quantum Number (n): Describes the energy level and size of the orbital.
- Angular Momentum Quantum Number (l): Describes the shape of the orbital.
- Magnetic Quantum Number (ml): Describes the orientation of the orbital in space. For s orbitals (l=0), ml is always 0, indicating only one possible orientation.
- Spin Quantum Number (ms): Describes the intrinsic angular momentum, or spin, of the electron. It can have only two values: +1/2 (spin up) or -1/2 (spin down).
Applying the Pauli Exclusion Principle to the s Orbital
Because the s orbital has only one possible value for n, l, and ml, the only way to distinguish between electrons within the s orbital is through their spin quantum number (ms). Since ms can be either +1/2 or -1/2, an s orbital can accommodate a maximum of two electrons, one with spin up and one with spin down.
Beyond the Single s Orbital: Shells and Subshells
While a single s orbital can hold only two electrons, atoms possess multiple energy levels (shells) and subshells. Each shell contains one s orbital, and successive shells contain additional orbitals of higher angular momentum (like p, d, and f orbitals).
Building Up the Electron Configuration: The Aufbau Principle
The Aufbau principle guides the filling of orbitals with electrons. Electrons fill orbitals starting with the lowest energy level and working their way up. This means that the 1s orbital is filled before the 2s orbital, the 2s orbital before the 2p orbitals, and so on.
Hund's Rule: Maximizing Unpaired Electrons
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion. While this rule primarily applies to p, d, and f orbitals, it's important to understand the overall context of electron filling.
Examples: Electron Configurations of Elements
Let's look at a few examples to illustrate the filling of s orbitals:
- Hydrogen (H): Has one electron, which occupies the 1s orbital (1s<sup>1</sup>).
- Helium (He): Has two electrons, both occupying the 1s orbital (1s<sup>2</sup>). This completely fills the 1s orbital.
- Lithium (Li): Has three electrons. Two occupy the 1s orbital, and the third occupies the 2s orbital (1s<sup>2</sup>2s<sup>1</sup>).
- Beryllium (Be): Has four electrons. Two occupy the 1s orbital, and two occupy the 2s orbital (1s<sup>2</sup>2s<sup>2</sup>). This completely fills the 2s orbital.
These examples showcase how s orbitals are filled systematically according to the Aufbau principle and Hund's rule.
Consequences of s Orbital Electron Occupancy: Chemical Properties
The number of electrons in the s orbital significantly influences the chemical properties of an element. The outermost electrons, often residing in s and p orbitals, are known as valence electrons, and they directly participate in chemical bonding.
Alkali Metals and Alkaline Earth Metals
Alkali metals (like lithium and sodium) have one electron in their outermost s orbital, making them highly reactive. They readily lose this electron to form a +1 ion, achieving a stable electron configuration. Alkaline earth metals (like beryllium and magnesium) have two electrons in their outermost s orbital and tend to lose these electrons to form +2 ions.
Noble Gases and Chemical Inertness
Noble gases (like helium and neon) have completely filled outermost s orbitals (and often p orbitals as well). This full electron configuration renders them chemically inert, as they have little tendency to gain or lose electrons. Their stability stems directly from the complete filling of the s orbital and other outer orbitals.
Conclusion: The Significance of the s Orbital
The seemingly simple s orbital plays a pivotal role in determining the properties of elements. Its spherical shape, capacity to hold two electrons, and its central position in the electron configuration of atoms contribute significantly to an element's reactivity, bonding behavior, and overall chemical characteristics. Understanding the nuances of the s orbital, including its quantum numbers and the principles governing electron occupancy, offers a fundamental understanding of atomic structure and the periodic trends observed in the elements. From the reactive alkali metals to the inert noble gases, the story of chemical behavior begins with the two electrons comfortably residing within the spherical embrace of the s orbital.
Latest Posts
Latest Posts
-
How Many Oxygen Molecules Can One Hemoglobin Carry
Mar 18, 2025
-
Which Of The Following Is Not A Form Of Precipitation
Mar 18, 2025
-
Which Statement About Natural Selection Is True
Mar 18, 2025
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The S Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
