How Many Electrons Are In Br
News Leon
Mar 18, 2025 · 5 min read
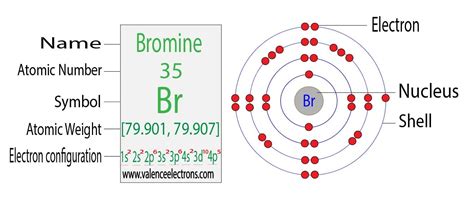
Table of Contents
How Many Electrons Are in Br? Understanding Atomic Structure and Electron Configuration
Determining the number of electrons in a bromine atom (Br) requires a fundamental understanding of atomic structure and the periodic table. This article will delve into the intricacies of atomic composition, explain how to determine the electron count for any element, and specifically address the electron configuration of bromine. We'll also explore related concepts to provide a comprehensive understanding of this essential chemical concept.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Atoms are the fundamental building blocks of matter. They consist of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and determines its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles that orbit the nucleus in energy levels or shells. The number of electrons typically equals the number of protons in a neutral atom, ensuring an overall neutral charge.
The Periodic Table: A Key to Atomic Information
The periodic table is a crucial tool for determining the number of electrons in an atom. Each element on the table is assigned an atomic number, which represents the number of protons (and, in a neutral atom, electrons) in its nucleus. Bromine (Br) is found in group 17 (or VIIA), period 4 of the periodic table. Its atomic number is 35.
Determining the Number of Electrons in Bromine (Br)
Since the atomic number of bromine is 35, a neutral bromine atom contains 35 electrons. This is because the number of protons (positive charges) and electrons (negative charges) are equal in a neutral atom, resulting in a balanced charge.
Electron Configuration: Understanding Electron Arrangement
The arrangement of electrons within an atom's energy levels is described by its electron configuration. This configuration dictates the atom's chemical properties and reactivity. For bromine, the electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
Let's break down this configuration:
- 1s²: The first energy level (n=1) contains one subshell, 's', which can hold up to two electrons. Bromine has two electrons in this subshell.
- 2s² 2p⁶: The second energy level (n=2) contains an 's' subshell (holding two electrons) and a 'p' subshell (holding six electrons). Together, they hold eight electrons.
- 3s² 3p⁶: The third energy level (n=3) also has 's' and 'p' subshells, holding a total of eight electrons.
- 4s² 3d¹⁰ 4p⁵: The fourth energy level (n=4) is more complex. It includes 's', 'd', and 'p' subshells. The 's' subshell holds two electrons, the 'd' subshell holds ten, and the 'p' subshell holds five electrons. This adds up to 17 electrons.
Adding the electrons in each subshell (2 + 8 + 8 + 17 = 35) confirms that a neutral bromine atom has 35 electrons.
Ions and Electron Count: The Role of Charge
It's important to remember that the number of electrons can change when an atom forms an ion. Ions are charged particles formed when atoms gain or lose electrons.
- Anions: Atoms that gain electrons become negatively charged anions. For example, a bromide ion (Br⁻) has gained one electron, resulting in a total of 36 electrons.
- Cations: Atoms that lose electrons become positively charged cations. Bromine rarely forms cations, as it is highly electronegative and prefers to gain electrons.
Bromine's Chemical Properties and Electron Configuration
Bromine's chemical properties are directly related to its electron configuration, specifically the five electrons in its 4p subshell. This incomplete outer shell makes bromine highly reactive. It readily gains one electron to achieve a stable octet (eight electrons in its outermost shell), forming the bromide ion (Br⁻). This tendency to gain an electron is responsible for bromine's role in various chemical reactions and compounds.
Valence Electrons: The Key to Reactivity
Valence electrons are the electrons in the outermost shell of an atom. They are the electrons involved in chemical bonding and determine the atom's reactivity. In bromine, the valence electrons are the seven electrons in the 4s and 4p subshells (4s² 4p⁵). This is why bromine readily forms one covalent bond or gains one electron to complete its octet.
Isotopes and Electron Count: The Influence of Neutrons
While the number of electrons in a neutral atom is determined by the atomic number, it's important to acknowledge the existence of isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. Different isotopes of bromine will have different mass numbers (protons + neutrons) but the same number of electrons (35) in their neutral state.
Applications of Bromine and its Compounds
Bromine and its compounds have numerous applications in various industries:
- Flame Retardants: Brominated flame retardants are used in plastics, textiles, and other materials to reduce their flammability.
- Water Treatment: Bromine compounds are employed as disinfectants in swimming pools and spas.
- Agriculture: Certain bromine compounds are used as fumigants and pesticides.
- Pharmaceuticals: Bromine is found in some pharmaceuticals and medicinal compounds.
- Photography: Bromine compounds have historical use in photographic processes.
Conclusion: Understanding Bromine's Electron Count and its Significance
Determining the number of electrons in bromine, a seemingly simple task, opens a window into the fascinating world of atomic structure and chemical behavior. The 35 electrons in a neutral bromine atom are not randomly distributed; their arrangement within specific energy levels and subshells dictates bromine's reactivity and its role in numerous compounds and applications. Understanding electron configuration and valence electrons is crucial for comprehending the chemical properties of all elements and their interactions within the broader context of chemistry. The detailed explanation provided here should solidify your understanding of bromine's electron count and its importance in the realm of chemistry and beyond.
Latest Posts
Latest Posts
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
-
How Many Water Molecules In A Drop Of Water
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In Br . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
