How Many Atoms Are In H2so4
News Leon
Mar 30, 2025 · 5 min read
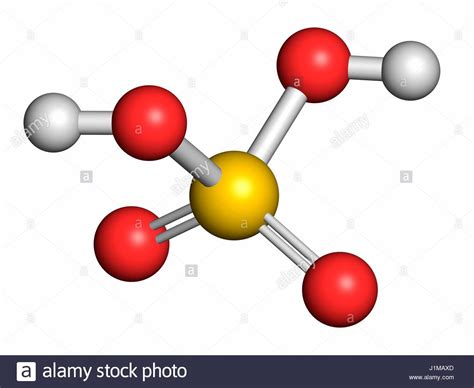
Table of Contents
How Many Atoms are in H₂SO₄? A Deep Dive into Molecular Composition
Understanding the composition of molecules is fundamental to chemistry. This article will delve into the seemingly simple question: how many atoms are in H₂SO₄ (sulfuric acid)? We'll explore not just the answer, but also the underlying concepts, the significance of chemical formulas, and how this knowledge applies to broader chemical principles.
Deconstructing the Chemical Formula: H₂SO₄
The chemical formula H₂SO₄ provides a concise and powerful representation of sulfuric acid's composition. Let's break it down:
- H: This symbol represents the element hydrogen.
- 2: The subscript '2' indicates that there are two atoms of hydrogen in each molecule of sulfuric acid.
- S: This symbol represents the element sulfur. There is one sulfur atom per molecule.
- O: This symbol represents the element oxygen.
- 4: The subscript '4' indicates that there are four atoms of oxygen in each molecule of sulfuric acid.
Therefore, by simply examining the formula, we can definitively answer the initial question: a molecule of H₂SO₄ contains a total of 7 atoms. This is the sum of 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms.
Beyond the Simple Count: Understanding Molecular Structure
While knowing the total atom count is important, understanding the arrangement of these atoms within the molecule is crucial to grasping sulfuric acid's properties and reactivity. H₂SO₄ doesn't exist as a simple linear arrangement of atoms. Instead, it possesses a more complex tetrahedral structure around the central sulfur atom.
The Tetrahedral Structure
The sulfur atom is at the center, bonded to two hydroxyl (-OH) groups and two oxygen atoms. These bonds are not all identical; some are single bonds (covalent bonds sharing one electron pair) and some are double bonds (covalent bonds sharing two electron pairs). This difference in bonding contributes significantly to sulfuric acid's strong acidity.
Implications of Molecular Structure
The structure of H₂SO₄ directly influences its properties:
- Acidity: The presence of two hydroxyl groups readily donate protons (H⁺ ions), making sulfuric acid a strong diprotic acid. This means it can donate two protons per molecule in aqueous solution.
- Reactivity: The double bonds between sulfur and oxygen contribute to the molecule's high reactivity. This allows it to participate in a wide range of chemical reactions, including esterification, dehydration, and sulfonation.
- Solubility: The polar nature of the molecule, due to the electronegativity differences between the atoms, results in its high solubility in water.
Avogadro's Number and Moles: Scaling Up from One Molecule
While we've determined the number of atoms in a single H₂SO₄ molecule, it's often necessary to work with macroscopic quantities of the substance. This is where Avogadro's number comes into play.
Avogadro's Number: A Chemical Constant
Avogadro's number (approximately 6.022 x 10²³) represents the number of entities (atoms, molecules, ions, etc.) in one mole of a substance. A mole is a unit of measurement in chemistry, analogous to a dozen (12) or a gross (144).
Moles of H₂SO₄ and Atom Calculation
If we have one mole of H₂SO₄, we know that there are 6.022 x 10²³ molecules present. Since each molecule contains 7 atoms, the total number of atoms in one mole of H₂SO₄ is:
7 atoms/molecule * 6.022 x 10²³ molecules/mole = 4.215 x 10²⁴ atoms/mole
This calculation demonstrates how Avogadro's number allows us to scale up from the microscopic level (individual molecules) to the macroscopic level (moles).
Applications of Sulfuric Acid: A Versatile Compound
The unique properties of sulfuric acid stem from its molecular composition and structure. Its widespread industrial applications are a testament to its versatility:
- Fertilizer Production: Sulfuric acid is crucial in the production of phosphate fertilizers, which are essential for agriculture.
- Petroleum Refining: It plays a vital role in the refining of crude oil, facilitating processes like alkylation and isomerization.
- Metal Processing: Used in various stages of metal processing, from ore extraction to surface treatment.
- Battery Manufacturing: A key component in lead-acid batteries, a widely used energy storage technology.
- Chemical Synthesis: Serves as a catalyst and reactant in numerous chemical syntheses, contributing to the production of various chemicals and materials.
Beyond H₂SO₄: Extending the Concept to Other Molecules
The principles we've discussed for calculating the number of atoms in H₂SO₄ can be applied to any chemical compound. Understanding the chemical formula is the key:
- Identify the elements present.
- Determine the number of atoms of each element from the subscripts.
- Sum the total number of atoms.
For example:
- Water (H₂O): Contains 2 hydrogen atoms + 1 oxygen atom = 3 atoms total
- Methane (CH₄): Contains 1 carbon atom + 4 hydrogen atoms = 5 atoms total
- Glucose (C₆H₁₂O₆): Contains 6 carbon atoms + 12 hydrogen atoms + 6 oxygen atoms = 24 atoms total
Conclusion: The Significance of Atomic Composition
The seemingly simple question of how many atoms are in H₂SO₄ leads us to a deeper understanding of molecular composition, chemical formulas, Avogadro's number, and the importance of molecular structure in determining the properties and applications of chemical compounds. From the microscopic realm of individual atoms to the macroscopic scale of industrial applications, the concept of atomic composition remains central to the field of chemistry. This knowledge forms a bedrock upon which further exploration of chemical reactions, properties, and applications can be built. Understanding this fundamental concept opens doors to comprehending the complex world of chemical interactions and their impact on our lives. The ability to calculate the number of atoms in any given molecule is not only a useful skill in chemistry, but also a crucial stepping stone in understanding more advanced chemical concepts.
Latest Posts
Latest Posts
-
Are Cells Depicted Plant Or Animal
Apr 01, 2025
-
Select Which Statements Are A Part Of Natural Selection
Apr 01, 2025
-
Why Electronic Configuration Of Calcium Is 2 8 8 2
Apr 01, 2025
-
1 Square Meter Is How Many Square Centimeters
Apr 01, 2025
-
Distance Between A Line And Plane
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
