How Does Reactivity Of Metals Increase
News Leon
Mar 18, 2025 · 5 min read
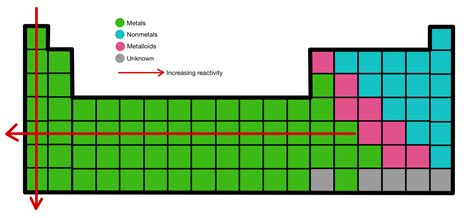
Table of Contents
How Does the Reactivity of Metals Increase? Understanding the Periodic Trends
The reactivity of metals, a fundamental concept in chemistry, dictates their behavior in various chemical reactions. Understanding what influences this reactivity is crucial for predicting reaction outcomes and designing numerous applications, from batteries to construction materials. This comprehensive guide delves into the factors determining metal reactivity, explaining how it increases across the periodic table and within specific groups.
The Electrochemical Series: A Key to Understanding Reactivity
The electrochemical series, a list of metals arranged in order of their decreasing reactivity, serves as a cornerstone for understanding this topic. Highly reactive metals readily lose electrons to form positive ions (cations), while less reactive metals are less inclined to do so. This electron loss is the essence of metallic reactivity. The higher a metal sits on the electrochemical series, the more readily it gives up its electrons.
Why Do Some Metals React More Readily Than Others?
The key lies in the electronic structure of the atoms. Specifically, these factors heavily influence a metal's reactivity:
-
Ionization Energy: This represents the energy required to remove an electron from a neutral atom. Lower ionization energy implies greater ease in losing electrons, thus higher reactivity. Alkali metals, for example, have exceptionally low ionization energies, explaining their high reactivity.
-
Electron Shielding: Inner electrons shield outer electrons from the attractive force of the nucleus. Increased shielding reduces the effective nuclear charge experienced by outer electrons, making them easier to remove and leading to increased reactivity.
-
Atomic Radius: As you move down a group in the periodic table, atomic radius increases. This means the outermost electrons are further from the nucleus, experiencing weaker attraction. Consequently, they are more readily lost, increasing reactivity.
-
Electro negativity: This measures the tendency of an atom to attract electrons towards itself. Metals generally have low electronegativity, meaning they are more likely to lose electrons rather than gain them, enhancing their reactivity.
Periodic Trends: Moving Across and Down the Table
The periodic table elegantly organizes elements based on their properties, including reactivity. Let's examine how reactivity changes across periods and down groups:
Reactivity Increases Down a Group
As you move down a group (e.g., Group 1, the alkali metals), reactivity increases significantly. This is primarily due to the increasing atomic radius and increased electron shielding. The outermost electron is progressively further from the nucleus, experiencing weaker attraction and hence being more easily lost.
Example: Lithium (Li) is less reactive than Sodium (Na), which is less reactive than Potassium (K), and so on. This trend continues down the alkali metal group.
Reactivity Generally Decreases Across a Period
Moving from left to right across a period (e.g., Period 3), reactivity generally decreases. This is because the effective nuclear charge increases while the atomic radius decreases. The increased nuclear charge attracts the outer electrons more strongly, making them harder to remove. The addition of protons to the nucleus outweighs the addition of electrons to the same shell, leading to a tighter hold on the electrons.
Example: Sodium (Na) is significantly more reactive than Magnesium (Mg), which is more reactive than Aluminum (Al), and so on. The trend continues across the period until the non-metals are reached.
Specific Examples: Illustrating Reactivity Differences
Let's consider some specific examples to highlight the differences in reactivity among various metals:
Alkali Metals (Group 1): The Most Reactive Metals
Alkali metals, located in Group 1, are known for their extremely high reactivity. This is due to their single valence electron, which is easily lost to form a +1 ion. They react vigorously with water, producing hydrogen gas and a metal hydroxide. The reaction becomes even more vigorous as you move down the group.
Example: Lithium reacts with water relatively slowly, while sodium reacts violently, and potassium reacts explosively. This demonstrates the increasing reactivity down the group.
Alkaline Earth Metals (Group 2): Moderately Reactive Metals
Alkaline earth metals, found in Group 2, are less reactive than alkali metals. They have two valence electrons, which are relatively easy to lose, forming +2 ions. They also react with water, although generally less vigorously than alkali metals. Reactivity increases down the group, similar to alkali metals.
Example: Magnesium reacts slowly with water, while calcium reacts more readily, and strontium even more so.
Transition Metals: Variable Reactivity
Transition metals exhibit more variable reactivity compared to alkali and alkaline earth metals. Their reactivity is influenced by several factors, including their electronic configuration, oxidation states, and the presence of d-orbitals. Some transition metals are relatively unreactive, while others are quite reactive.
Example: Gold (Au) is notoriously unreactive, while iron (Fe) is relatively reactive and readily rusts in the presence of oxygen and water.
Factors Influencing Reactivity Beyond Periodic Trends
While the periodic trends provide a general framework for understanding metal reactivity, other factors can influence it:
-
Surface Area: A larger surface area increases the contact between the metal and reactants, accelerating the reaction rate. Finely divided metals react much faster than solid chunks.
-
Temperature: Higher temperatures generally increase reaction rates by providing more kinetic energy for the reacting particles to overcome activation energy barriers.
-
Concentration of Reactants: Higher concentrations of reactants increase the probability of collisions between reacting species, leading to faster reactions.
-
Presence of Catalysts: Catalysts can significantly increase reaction rates by lowering the activation energy required for the reaction to proceed.
Applications of Understanding Metal Reactivity
Understanding the reactivity of metals has wide-ranging applications in various fields:
-
Metallurgy: The extraction of metals from their ores heavily relies on understanding their relative reactivity. More reactive metals require more energy-intensive processes for extraction.
-
Corrosion: Understanding reactivity helps in predicting and preventing corrosion, a significant problem in many applications. Protective coatings and sacrificial anodes are used to minimize corrosion.
-
Battery Technology: The reactivity of metals plays a critical role in the development of batteries. Different metals with varying reactivity are used as electrodes to generate electrical energy.
-
Chemical Synthesis: The reactivity of metals is crucial in many chemical reactions, enabling the synthesis of various compounds and materials.
Conclusion: A Deeper Understanding of Reactivity
Metal reactivity is a complex phenomenon determined by a combination of factors, primarily rooted in the electronic structure of the metal atoms. Understanding the periodic trends and other influencing factors is vital for predicting reaction behavior and utilizing metals effectively in various applications. By mastering this concept, we can unlock the potential of metals in countless technological advancements and innovations. Further research and exploration continue to refine our understanding of these complex interactions, driving progress in various scientific disciplines.
Latest Posts
Latest Posts
-
What Is The Product Of The Following Sequence Of Reactions
Mar 19, 2025
-
How Are Weathering And Erosion Difference
Mar 19, 2025
-
Double Replacement Examples In Real Life
Mar 19, 2025
-
The Study Of Population Is Called
Mar 19, 2025
-
Oxidation Number Of O In H2o
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Does Reactivity Of Metals Increase . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
