How Does Branching Affect Boiling Point
News Leon
Mar 29, 2025 · 5 min read
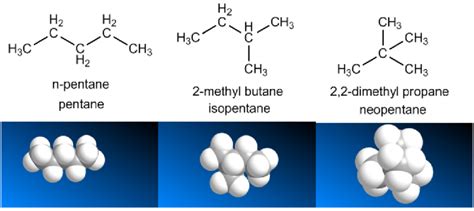
Table of Contents
How Does Branching Affect Boiling Point? Understanding the Impact of Molecular Structure
Boiling point, a fundamental physical property of liquids, represents the temperature at which a substance transitions from its liquid state to its gaseous state. This transition occurs when the vapor pressure of the liquid equals the surrounding atmospheric pressure. While numerous factors influence boiling point, the molecular structure, particularly the presence and extent of branching, plays a significant role. This article delves into the intricate relationship between branching and boiling point, exploring the underlying principles and providing illustrative examples.
The Interplay of Intermolecular Forces and Branching
The boiling point of a substance is directly related to the strength of the intermolecular forces (IMFs) holding its molecules together. Stronger IMFs require more energy to overcome, resulting in a higher boiling point. The primary types of IMFs include:
- London Dispersion Forces (LDFs): Present in all molecules, these forces arise from temporary fluctuations in electron distribution, creating instantaneous dipoles. LDF strength increases with the size and surface area of the molecule.
- Dipole-Dipole Interactions: Occur in polar molecules possessing permanent dipoles due to differences in electronegativity between atoms. These forces are stronger than LDFs.
- Hydrogen Bonding: A special type of dipole-dipole interaction involving hydrogen bonded to a highly electronegative atom (oxygen, nitrogen, or fluorine). Hydrogen bonds are the strongest type of IMF.
Branching significantly alters the shape and surface area of a molecule, directly affecting the strength of its IMFs, particularly LDFs.
How Branching Reduces Boiling Point
The impact of branching on boiling point is predominantly observed through its effect on London Dispersion Forces. Let's consider the following:
1. Reduced Surface Area:
Linear molecules exhibit a larger surface area compared to their branched counterparts. A greater surface area allows for more points of contact between molecules, leading to stronger LDFs and a higher boiling point. Branching compacts the molecule, reducing its surface area and consequently weakening the LDFs.
Imagine two hydrocarbons: n-pentane (a straight-chain alkane) and neopentane (a highly branched isomer). n-pentane, with its extended structure, has a larger surface area for intermolecular interactions, resulting in a higher boiling point (36.1 °C) than neopentane (9.5 °C).
2. Decreased Molecular Polarizability:
Branching affects the distribution of electrons within the molecule. In branched molecules, the electrons are less easily polarized, meaning they are less susceptible to forming temporary dipoles. This reduced polarizability leads to weaker LDFs and a lower boiling point.
3. Hindrance to Packing:
Branched molecules are less efficiently packed in the liquid phase compared to linear molecules. This inefficient packing reduces the number of effective intermolecular contacts and weakens the overall LDFs, lowering the boiling point. The more compact structure hinders the ability of molecules to approach each other closely, thus minimizing the attractive forces.
Examples Illustrating the Branching Effect
Let's examine several examples to further solidify the relationship between branching and boiling point:
Alkanes: As the number of carbon atoms in an alkane increases, the boiling point generally increases due to the increasing strength of LDFs. However, introducing branching significantly lowers the boiling point. For instance:
- Butane (C4H10): n-butane (linear) boils at -0.5 °C, while isobutane (branched) boils at -11.7 °C.
- Pentane (C5H12): n-pentane boils at 36.1 °C, isopentane at 27.7 °C, and neopentane at 9.5 °C. The increasing degree of branching correlates with a decrease in boiling point.
Alcohols: While hydrogen bonding dominates the intermolecular forces in alcohols, branching still influences the boiling point. The branched alcohols have lower boiling points compared to their linear isomers due to a reduction in the effectiveness of hydrogen bonding and weaker LDFs. For example:
- Butanol (C4H9OH): n-butanol boils at 117.7 °C, while isobutanol boils at 107.9 °C, and tert-butanol boils at 82.2 °C. The higher degree of branching in tert-butanol drastically reduces its boiling point.
Factors Beyond Branching: A Holistic View
While branching is a crucial factor, it's essential to acknowledge that other structural features influence boiling point. These include:
- Molecular Weight: Higher molecular weight generally correlates with higher boiling points due to increased LDFs.
- Polarity: Polar molecules exhibit higher boiling points than nonpolar molecules of comparable size due to dipole-dipole interactions.
- Hydrogen Bonding: The presence of hydrogen bonding significantly elevates the boiling point due to the strong attractive forces.
Therefore, the boiling point is a complex interplay of these factors, with branching playing a significant role in modifying the strength of IMFs, primarily LDFs.
Predicting Boiling Points: Practical Applications
Understanding the influence of branching on boiling point has practical applications in various fields:
- Chemical Engineering: Predicting boiling points is vital for designing efficient distillation processes, crucial for separating mixtures of organic compounds. The knowledge of branching effects aids in optimizing separation techniques.
- Material Science: The boiling points of substances dictate their suitability for various applications. Understanding the influence of branching can help select materials with specific properties, such as volatility or thermal stability.
- Petroleum Refining: The branching in hydrocarbons significantly affects their properties, influencing the efficiency of refining processes and the quality of fuel products.
Conclusion: A Deeper Understanding of Branching's Impact
The effect of branching on boiling point is a compelling illustration of how subtle changes in molecular structure can profoundly impact macroscopic properties. The reduction in surface area, decreased molecular polarizability, and hindered packing caused by branching all contribute to weaker intermolecular forces, leading to lower boiling points. This relationship is critical in various scientific and engineering disciplines, emphasizing the importance of understanding molecular structure and its consequences. By grasping the intricate interplay between branching and IMFs, we gain a deeper appreciation for the fundamental principles governing the physical properties of liquids and their applications in the real world. Future research continues to refine our understanding of these complex relationships, leading to further advancements in various scientific and technological fields. Further exploration into the effects of different types and degrees of branching, alongside other structural features, will yield a more comprehensive understanding of boiling point prediction and its relevance to diverse applications.
Latest Posts
Latest Posts
-
Carbon Dioxide Is Held Together By This Type Of Bond
Apr 01, 2025
-
What Is The Product Of The Reaction Shown Below
Apr 01, 2025
-
Is Internal Energy Intensive Or Extensive
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Does Branching Affect Boiling Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
