Carbon Dioxide Is Held Together By This Type Of Bond
News Leon
Apr 01, 2025 · 6 min read
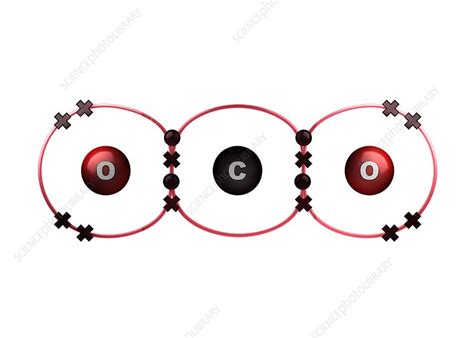
Table of Contents
Carbon Dioxide: A Deep Dive into its Bonding and Properties
Carbon dioxide (CO₂) is a ubiquitous molecule, playing a crucial role in various natural processes and human activities. Understanding its structure and the type of bonds holding it together is fundamental to grasping its behavior and impact on our environment. This article delves into the intricacies of carbon dioxide's bonding, exploring its properties and significance. We will explore the covalent bonds that form the backbone of this essential molecule.
The Covalent Bond: Sharing is Caring
At the heart of CO₂'s structure lies the covalent bond, a type of chemical bond where atoms share electrons to achieve a stable electron configuration. Unlike ionic bonds, where electrons are transferred from one atom to another, covalent bonds involve the mutual sharing of electrons between atoms. This sharing creates a strong attraction that holds the atoms together, forming a molecule.
Understanding Electron Shells and Octet Rule
To understand covalent bonding in CO₂, let's consider the electronic structure of the constituent atoms: carbon (C) and oxygen (O). Carbon has four electrons in its outermost shell (valence shell), while oxygen has six. Atoms strive to achieve a stable electron configuration, often following the octet rule, which states that atoms tend to gain, lose, or share electrons to have eight electrons in their valence shell (except for hydrogen and helium, which strive for two).
Formation of Covalent Bonds in CO₂
In CO₂, the carbon atom shares two electrons with each of the two oxygen atoms. Each oxygen atom, in turn, shares two electrons with the carbon atom. This sharing results in the formation of two double covalent bonds between the central carbon atom and each oxygen atom. Each oxygen atom now effectively has eight electrons in its valence shell (two from its own lone pairs and six from the shared electrons), fulfilling the octet rule. Similarly, the carbon atom attains a complete octet with eight shared electrons.
The Linear Structure of CO₂
The double covalent bonds between carbon and oxygen atoms in CO₂ dictate its linear molecular geometry. This means the three atoms are arranged in a straight line, with the carbon atom at the center and the two oxygen atoms on either side. This linear arrangement minimizes electron-electron repulsion and contributes to the molecule's stability.
Bond Length and Bond Strength
The double bonds in CO₂ are relatively strong and short compared to single bonds. The bond length, the distance between the nuclei of two bonded atoms, is shorter for double bonds because of the increased electron density between the atoms. This shorter bond length translates to a stronger bond, requiring more energy to break the bond.
Properties of CO₂ Influenced by Bonding
The unique bonding in CO₂ directly influences its physical and chemical properties.
Nonpolar Nature
Despite the presence of polar C=O bonds (oxygen is more electronegative than carbon, leading to a slight charge separation), the linear geometry of CO₂ results in a nonpolar molecule. The dipole moments of the two C=O bonds are equal in magnitude and opposite in direction, effectively canceling each other out. This nonpolar nature affects CO₂'s solubility and interactions with other molecules.
Gas at Room Temperature
The relatively weak intermolecular forces (London Dispersion Forces) between CO₂ molecules, a consequence of its nonpolar nature, lead to its existence as a gas at room temperature. The weak attractions between molecules allow them to move freely, resulting in a gaseous state.
Solubility in Water
While CO₂ is sparingly soluble in water, its solubility increases under pressure. The dissolution of CO₂ in water results in the formation of carbonic acid (H₂CO₃), a weak acid that contributes to the acidity of rainwater and plays a critical role in the carbon cycle.
The Significance of CO₂ in the Environment and Human Activities
Carbon dioxide plays a pivotal role in several environmental processes and human activities:
The Greenhouse Effect
CO₂ is a significant greenhouse gas, trapping heat in the Earth's atmosphere and contributing to the greenhouse effect. While the greenhouse effect is essential for maintaining a habitable temperature on Earth, increased CO₂ levels due to human activities (e.g., burning fossil fuels, deforestation) enhance the effect, leading to global warming and climate change.
Photosynthesis
Plants use CO₂ as a source of carbon in photosynthesis, the process by which they convert light energy into chemical energy in the form of glucose. CO₂ is crucial for plant growth and is a fundamental component of the global carbon cycle.
Carbon Cycle
CO₂ is a central component of the carbon cycle, a biogeochemical cycle where carbon atoms are exchanged between the atmosphere, oceans, land, and living organisms. Human activities have significantly disrupted the carbon cycle, leading to an increase in atmospheric CO₂ concentrations.
Industrial Applications
CO₂ has various industrial applications, including as a refrigerant, a supercritical fluid in various processes, and a component in the production of certain chemicals. Its properties, dictated by its bonding, make it suitable for these applications.
Beyond the Basics: Resonance and Hybridization
A more advanced understanding of CO₂'s bonding involves the concepts of resonance and hybridization.
Resonance
The double bonds in CO₂ aren't fixed; instead, they exhibit resonance. This means the electrons in the double bonds are delocalized, meaning they are not confined to a single location but are spread out across the molecule. This delocalization contributes to the stability of the CO₂ molecule. We can represent this with two resonance structures, each showing one double bond and one single bond between carbon and oxygen atoms, but the actual molecule is a hybrid of both structures.
Hybridization
The carbon atom in CO₂ undergoes sp hybridization. This means one s orbital and two p orbitals combine to form three sp hybrid orbitals, which are oriented linearly. These hybrid orbitals form sigma bonds with the oxygen atoms. The remaining p orbitals on the carbon and oxygen atoms overlap to form pi bonds, contributing to the double bond character of each C=O bond.
Conclusion: The Importance of Understanding CO₂ Bonding
Understanding the covalent bonding in CO₂, its linear structure, and the resulting properties is crucial for comprehending its role in various natural and industrial processes. The strength of its double bonds, its nonpolar nature, and its involvement in the greenhouse effect all stem from the fundamental arrangement of atoms and the sharing of electrons. Continued research into CO₂'s behavior and interactions with other molecules remains vital in addressing challenges related to climate change and harnessing its potential applications. The seemingly simple molecule of carbon dioxide, held together by covalent bonds, offers a rich tapestry of scientific insights and practical implications. Its significance in the world around us cannot be overstated.
Latest Posts
Latest Posts
-
Which Statement About Exothermic Reactions Is Accurate
Apr 02, 2025
-
A Lens Produces A Real Image Of A Real Object
Apr 02, 2025
-
Explain Common Different And Conflicting Goals By Giving Appropriate Examples
Apr 02, 2025
-
What Is The Major Product For The Following Reaction Sequence
Apr 02, 2025
-
Decomposers Are Important In The Environment Because They
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Carbon Dioxide Is Held Together By This Type Of Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
