How Do You Separate Nitrogen And Oxygen
News Leon
Apr 01, 2025 · 7 min read
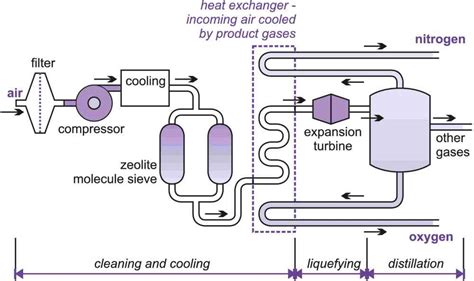
Table of Contents
How Do You Separate Nitrogen and Oxygen?
Air, the lifeblood of our planet, is a seemingly simple substance. However, a closer look reveals a complex mixture of gases, primarily nitrogen (approximately 78%) and oxygen (approximately 21%). Separating these two crucial components is a vital process with numerous industrial applications, from producing breathable air in hospitals to creating inert atmospheres for manufacturing. But how do we achieve this separation? The answer lies in understanding the subtle differences in their physical properties and employing techniques that exploit these differences.
Understanding the Challenge: The Similarities of Nitrogen and Oxygen
Before diving into the separation methods, it's crucial to acknowledge the inherent difficulty. Nitrogen and oxygen are both diatomic gases (N₂ and O₂), meaning they exist as pairs of atoms. They share a similar atomic size and molecular weight, leading to very similar physical properties such as boiling and melting points. This similarity makes their separation a significant engineering challenge. We can't simply use techniques like filtering, as the molecules are too small to be caught by any physical filter.
Key Methods for Separating Nitrogen and Oxygen
Several methods have been developed to effectively separate nitrogen and oxygen, each leveraging different aspects of their physical properties. The most common methods include:
1. Fractional Distillation: The Industrial Workhorse
Fractional distillation is the most widely used industrial method for separating nitrogen and oxygen. This technique relies on the slight difference in boiling points between the two gases:
- Oxygen's boiling point: -183°C (-297°F)
- Nitrogen's boiling point: -196°C (-321°F)
While this difference might seem small, it's sufficient for effective separation when a large-scale process is employed. The process involves the following steps:
-
Liquefaction: Air is first compressed, cooled, and then liquefied. This involves removing impurities such as carbon dioxide and water vapor. The cooling process often involves using expansion turbines, which utilize the Joule-Thomson effect to achieve cryogenic temperatures.
-
Fractional Distillation Column: The liquefied air is then introduced into a tall, specialized column called a fractional distillation column. As the liquefied air rises through the column, its temperature gradually decreases. Because oxygen has a higher boiling point, it liquefies and condenses at a higher temperature than nitrogen.
-
Separation: Oxygen is collected as a liquid at a higher point in the column, while nitrogen, with its lower boiling point, remains gaseous for longer and is collected at a lower point. The column is meticulously designed to maintain a temperature gradient that facilitates the efficient separation of the two gases.
-
Purification: The collected nitrogen and oxygen are often subjected to further purification processes to remove any remaining traces of the other gas or other impurities.
Advantages of Fractional Distillation:
- High Efficiency: Fractional distillation is capable of producing high-purity nitrogen and oxygen.
- Scalability: The process can be easily scaled up to produce large quantities of both gases.
- Established Technology: It's a mature technology with well-established engineering practices.
Disadvantages of Fractional Distillation:
- High Energy Consumption: Liquefying air requires substantial energy input, making it an energy-intensive process.
- Complex Equipment: The process requires specialized and complex equipment, including compressors, heat exchangers, and distillation columns.
- Cryogenic Temperatures: Operating at cryogenic temperatures presents safety challenges and requires specialized materials.
2. Pressure Swing Adsorption (PSA): A More Energy-Efficient Alternative
Pressure swing adsorption (PSA) offers a more energy-efficient alternative to fractional distillation, especially for smaller-scale applications. This method utilizes specialized adsorbent materials (zeolites, activated carbon, etc.) that selectively adsorb one gas over another based on differences in their molecular properties:
-
Adsorption: Compressed air is passed through a bed of adsorbent material. The adsorbent preferentially adsorbs oxygen, leaving nitrogen to pass through.
-
Pressure Swing: Once the adsorbent becomes saturated with oxygen, the pressure is reduced. This causes the adsorbed oxygen to desorb, making the adsorbent available for another cycle. The process typically employs two or more adsorption beds, ensuring continuous operation.
-
Product Stream: The nitrogen stream that passes through the adsorbent bed is collected as the product, while the desorbed oxygen can also be collected.
Advantages of PSA:
- Lower Energy Consumption: PSA generally consumes less energy compared to fractional distillation.
- Compact Equipment: PSA units are more compact and easier to install than fractional distillation systems.
- Lower Capital Cost: The initial investment for a PSA system is often lower.
Disadvantages of PSA:
- Lower Purity: PSA typically produces nitrogen of lower purity compared to fractional distillation, although high-purity systems are available.
- Adsorbent Life: The adsorbent materials have a finite lifespan and require periodic replacement.
- Sensitivity to Impurities: The efficiency of PSA can be affected by impurities in the feed air.
3. Membrane Separation: A Simple Yet Effective Technique
Membrane separation is a relatively simple and energy-efficient technique that utilizes semi-permeable membranes to separate gases based on their molecular size and solubility. The membranes allow nitrogen to pass through more readily than oxygen, leading to a nitrogen-enriched stream and an oxygen-enriched stream.
-
Membrane Material: These membranes are typically made of polymers with carefully controlled pore sizes.
-
Pressure Difference: A pressure difference across the membrane drives the separation process.
-
Product Streams: The permeate stream (passing through the membrane) is enriched in nitrogen, while the retentate stream (remaining on the feed side) is enriched in oxygen.
Advantages of Membrane Separation:
- Simple and Compact: Membrane separation systems are relatively simple and compact.
- Lower Energy Consumption: The energy consumption is significantly lower compared to other methods.
- Low Maintenance: Membrane systems generally require less maintenance than other separation techniques.
Disadvantages of Membrane Separation:
- Lower Purity: Membrane separation typically yields lower purity nitrogen and oxygen compared to other methods.
- Membrane Fouling: Membranes can become fouled by impurities, reducing their efficiency.
- Limited Scalability: Scaling up membrane separation systems can be challenging.
4. Cryogenic Air Separation Units (CASUs): Large-Scale Production
Cryogenic air separation units (CASUs) are large-scale industrial plants designed for the mass production of nitrogen and oxygen. These plants typically employ a combination of air compression, cooling, and fractional distillation techniques to achieve high production rates and purity levels. The scale and complexity of CASUs make them ideal for supplying large industrial customers.
Applications of Separated Nitrogen and Oxygen
The separated nitrogen and oxygen find a wide range of applications in various industries:
-
Healthcare: Oxygen is essential for medical applications, including respiratory support and medical procedures. Nitrogen is used in cryopreservation and as a shielding gas in some medical procedures.
-
Industrial Manufacturing: Nitrogen is widely used as an inert gas in packaging (to prevent oxidation), chemical processing, and metal fabrication. Oxygen is used in welding, cutting, and various chemical processes.
-
Food Industry: Nitrogen is used for food preservation, packaging, and to create modified atmospheres to extend shelf life.
-
Electronics Industry: Nitrogen is used in the production of semiconductors and other electronic components.
-
Aerospace: Oxygen is a crucial component of rocket propellants. Nitrogen is used in various aerospace applications.
Choosing the Right Separation Method
The choice of separation method depends on various factors, including:
-
Scale of production: For large-scale production, fractional distillation is typically the most cost-effective option. For smaller-scale applications, PSA or membrane separation may be more suitable.
-
Purity requirements: Fractional distillation produces the highest purity products. If lower purity is acceptable, PSA or membrane separation can be used to reduce costs.
-
Energy consumption: PSA and membrane separation are generally more energy-efficient than fractional distillation.
-
Capital costs: The initial investment for different methods can vary significantly.
Conclusion: A Vital Process with Wide-Ranging Applications
Separating nitrogen and oxygen from air is a fundamental industrial process with far-reaching implications across various sectors. The choice of separation method is dictated by specific needs and constraints. Fractional distillation remains the workhorse for large-scale applications, while PSA and membrane separation offer energy-efficient alternatives for smaller-scale needs. Continued advancements in separation technologies are likely to lead to even more efficient and cost-effective methods in the future. The ongoing development of these processes reflects the importance of these two gases in sustaining modern life and industry.
Latest Posts
Latest Posts
-
Which Of The Following Is Chemically Inert Unreactive
Apr 02, 2025
-
The Standard Unit For Measuring Volume Is
Apr 02, 2025
-
Materials Like Rubber That Resist The Flow Of E
Apr 02, 2025
-
What Are The Units Of Conductance
Apr 02, 2025
-
What Is Mega In Scientific Notation
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Do You Separate Nitrogen And Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
