Heat Is A Measure Of _____________ _____________.
News Leon
Mar 15, 2025 · 6 min read
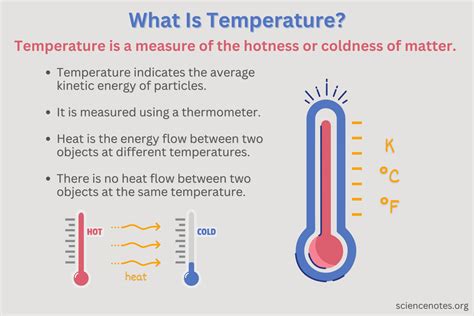
Table of Contents
Heat is a Measure of Average Kinetic Energy
Heat is a fundamental concept in physics and thermodynamics, often misunderstood and conflated with temperature. While related, they are distinct entities. A precise and complete definition reveals that heat is a measure of average kinetic energy. This article will delve deep into this definition, exploring the microscopic nature of heat, its relationship to temperature, and its implications in various physical phenomena. We'll also touch upon its practical applications and the significance of understanding this fundamental concept.
Understanding Kinetic Energy and its Relation to Heat
Before diving into the definition, let's establish a firm understanding of kinetic energy. Kinetic energy is the energy an object possesses due to its motion. The faster an object moves, the greater its kinetic energy. This is quantified by the formula KE = 1/2 * mv², where 'm' represents mass and 'v' represents velocity.
Now, consider a system composed of countless particles, like atoms or molecules, in a substance. These particles are in constant, random motion – vibrating, rotating, and translating. The kinetic energy of each individual particle contributes to the overall energy of the system. Heat, in essence, is the measure of the total kinetic energy of these particles, specifically the average kinetic energy.
It's crucial to emphasize the word "average." The particles within a substance don't all move at the same speed. Some move faster, some slower. Heat reflects the average of this kinetic energy across all the particles. A higher average kinetic energy signifies more heat, while a lower average signifies less heat.
Distinguishing Heat from Temperature
While closely linked, heat and temperature are not interchangeable. Temperature is a measure of the average kinetic energy of the particles in a system. Heat, on the other hand, is the total kinetic energy of the particles. The distinction is subtle but critical.
Imagine two containers, one containing a small amount of boiling water and the other containing a large amount of lukewarm water. The boiling water has a higher temperature, meaning the average kinetic energy of its particles is higher. However, the larger container of lukewarm water might possess more total kinetic energy – more heat – because it contains many more particles, even if their average kinetic energy is lower. This illustrates that a system can have a high temperature but relatively low heat, or vice versa.
Heat Transfer and its Mechanisms
Heat naturally flows from regions of higher temperature (higher average kinetic energy) to regions of lower temperature (lower average kinetic energy). This transfer continues until thermal equilibrium is reached, meaning both regions have the same average kinetic energy. Three primary mechanisms facilitate heat transfer:
1. Conduction: This occurs within a material or between materials in direct contact. The kinetic energy of faster-moving particles is transferred to slower-moving particles through collisions. Metals are excellent conductors due to their freely moving electrons, which readily transfer kinetic energy.
2. Convection: This mechanism involves the movement of fluids (liquids or gases). Warmer, less dense fluid rises, while cooler, denser fluid sinks, creating a convective current that transports heat. This is why heating a room with a radiator is effective; the warm air rises, forcing cooler air to circulate and get heated.
3. Radiation: This method doesn't require a medium; heat is transferred through electromagnetic waves. The sun's warmth reaching the Earth is a prime example of radiative heat transfer. All objects emit thermal radiation, the intensity of which depends on their temperature. Darker objects absorb and emit radiation more efficiently than lighter objects.
Measuring Heat: Units and Specific Heat Capacity
Heat is typically measured in joules (J), a unit of energy in the International System of Units (SI). However, other units, like calories (cal) and British Thermal Units (BTU), are also used. One calorie is defined as the amount of heat required to raise the temperature of one gram of water by one degree Celsius.
The specific heat capacity of a substance is a crucial property that quantifies the amount of heat required to raise the temperature of one unit of mass of that substance by one degree. Different substances have different specific heat capacities. Water, for instance, has a relatively high specific heat capacity, meaning it requires a significant amount of heat to change its temperature. This property makes water an excellent heat buffer, regulating temperature fluctuations in environments and organisms.
Heat and Phase Transitions
Heat plays a critical role in phase transitions, which are changes in the physical state of a substance (e.g., solid to liquid, liquid to gas). During phase transitions, heat is absorbed or released without a change in temperature. This heat is called latent heat.
For instance, when ice melts into water, the heat supplied doesn't increase the temperature; instead, it breaks the bonds holding the water molecules in a rigid structure, leading to a change in phase. Similarly, when water boils, the heat supplied is used to overcome the intermolecular forces holding the liquid together, leading to vaporization.
Applications of Heat and its Measurement
Understanding and manipulating heat is fundamental to numerous technological and industrial applications. Here are just a few:
- Power Generation: Heat from the combustion of fuels drives turbines in power plants, generating electricity.
- Heating and Cooling Systems: These systems rely on heat transfer principles to maintain comfortable indoor temperatures. Refrigerators use heat pumps to transfer heat from the inside to the outside.
- Manufacturing Processes: Many industrial processes, such as metal forging and plastic molding, require precise control of heat.
- Medical Applications: Heat therapy is used for various medical treatments, including pain relief and wound healing.
Advanced Concepts and Further Exploration
This article provides a foundational understanding of heat as a measure of average kinetic energy. However, the topic extends to more complex concepts in thermodynamics, including:
- Entropy: A measure of disorder or randomness in a system. Heat transfer tends to increase entropy.
- Enthalpy: A thermodynamic property that combines internal energy and pressure-volume work.
- Thermodynamic Laws: Fundamental laws governing energy and heat transfer.
Conclusion: The Significance of Understanding Heat
Understanding heat as a measure of average kinetic energy is essential for comprehending various physical phenomena and their applications. Its role in heat transfer, phase transitions, and numerous technologies underlines its fundamental importance in science and engineering. This knowledge lays the groundwork for further explorations into advanced concepts in thermodynamics and related fields, leading to innovations and breakthroughs in diverse areas. By grasping this fundamental concept, we gain a deeper appreciation for the intricate interplay of energy and matter in the world around us. Continued learning and exploration in this field will undoubtedly lead to more fascinating discoveries and practical advancements. The journey into the world of heat and thermodynamics is far from over, and there's always more to discover about this crucial aspect of our physical reality.
Latest Posts
Latest Posts
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
-
How Long Is A Thousand Days
Mar 17, 2025
-
How Many Valence Electrons In Copper
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Heat Is A Measure Of _____________ _____________. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
