Ground State Electron Configuration For Potassium
News Leon
Mar 15, 2025 · 6 min read
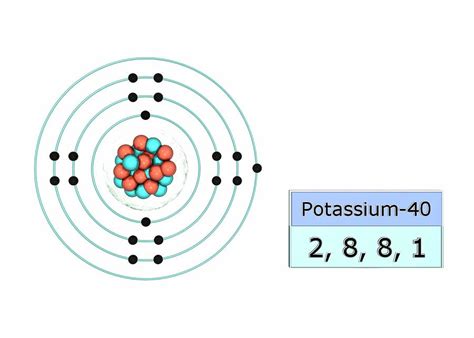
Table of Contents
Ground State Electron Configuration for Potassium: A Deep Dive
Potassium, a vital element for life, holds a fascinating place in the periodic table. Its relatively simple atomic structure, however, belies a rich tapestry of electron behavior that underpins its chemical properties and reactivity. Understanding the ground state electron configuration of potassium is key to unlocking this understanding. This comprehensive guide delves into the intricacies of potassium's electron arrangement, exploring the underlying principles, common notations, and implications for its chemical behavior.
Understanding Electron Configuration
Before diving into potassium's specific configuration, let's establish a foundational understanding of electron configuration itself. Electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement dictates how an atom will interact with other atoms, determining its chemical properties and reactivity. Electrons occupy orbitals, which are regions of space around the nucleus where there's a high probability of finding an electron. These orbitals are grouped into shells and subshells, characterized by their principal quantum number (n) and azimuthal quantum number (l), respectively.
Shells and Subshells
-
Shells (n): These represent the principal energy levels. The closer a shell is to the nucleus (smaller n), the lower its energy. We typically represent shells with numbers: n = 1, 2, 3, and so on.
-
Subshells (l): Within each shell are subshells, denoted by letters: s, p, d, and f. Each subshell contains a specific number of orbitals:
- s subshell: 1 orbital (holds up to 2 electrons)
- p subshell: 3 orbitals (holds up to 6 electrons)
- d subshell: 5 orbitals (holds up to 10 electrons)
- f subshell: 7 orbitals (holds up to 14 electrons)
The Aufbau principle, Hund's rule, and the Pauli exclusion principle guide the filling of these orbitals.
The Aufbau Principle: Filling Orbitals in Order of Increasing Energy
The Aufbau principle, meaning "building-up" in German, dictates that electrons fill orbitals in order of increasing energy. This isn't simply a matter of filling shells sequentially. The energy levels of subshells can overlap. A general order of filling is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p… and so on. This order is often depicted using a diagram called an Aufbau diagram or orbital filling diagram. However, it's important to remember that exceptions exist, particularly with transition metals and lanthanides/actinides.
Hund's Rule: Maximizing Unpaired Electrons
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This maximizes the total spin of the atom, leading to greater stability. Think of it as electrons preferring to "spread out" as much as possible within a subshell before pairing up.
The Pauli Exclusion Principle: A Maximum of Two Electrons per Orbital
The Pauli exclusion principle is fundamental to electron configuration. It states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms). This means that each orbital can hold a maximum of two electrons, and these electrons must have opposite spins (spin up, +1/2, and spin down, -1/2).
Determining the Ground State Electron Configuration of Potassium (K)
Potassium (K) has an atomic number of 19, meaning it has 19 protons and, in its neutral state, 19 electrons. To determine its ground state electron configuration, we follow the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
The filling order, following the Aufbau principle, proceeds as follows:
- 1s²: The first shell (n=1) contains only an s subshell, which can hold two electrons.
- 2s²: The second shell (n=2) starts with the 2s subshell, holding another two electrons.
- 2p⁶: The 2p subshell contains three orbitals, each holding two electrons, for a total of six.
- 3s²: The third shell (n=3) begins with the 3s subshell, holding two more electrons.
- 3p⁶: The 3p subshell, similar to 2p, holds six electrons.
- 4s¹: Finally, we reach the fourth shell (n=4). The 4s subshell receives the remaining electron.
Therefore, the complete ground state electron configuration of potassium is: 1s²2s²2p⁶3s²3p⁶4s¹.
Different Notations for Electron Configuration
Several notations can represent the same electron configuration:
-
Full Notation: This is the complete listing of all occupied orbitals, as shown above: 1s²2s²2p⁶3s²3p⁶4s¹.
-
Condensed Notation (Noble Gas Notation): This notation simplifies the configuration by using the symbol of the preceding noble gas in brackets to represent the inner core electrons. Since Argon (Ar) has the electron configuration 1s²2s²2p⁶3s²3p⁶, we can write potassium's configuration as: [Ar]4s¹. This notation is more compact and commonly used.
-
Orbital Diagram: This uses boxes to represent orbitals and arrows to represent electrons, clearly illustrating the electron spin. For potassium, the 4s orbital would have a single upward-pointing arrow.
The Significance of Potassium's 4s¹ Electron
The single electron in the 4s orbital is crucial to potassium's chemical behavior. This outermost electron, also known as the valence electron, is relatively loosely held compared to the inner electrons. This makes potassium highly reactive, readily losing this electron to form a +1 ion (K⁺). This ease of ionization is characteristic of alkali metals, the group to which potassium belongs. This reactivity explains potassium's role in various biological processes and its use in various chemical reactions.
Potassium's Reactivity and its Chemical Implications
Potassium's tendency to lose its outermost electron to achieve a stable octet (eight electrons in its outermost shell) is a driving force behind its reactivity. It readily reacts with water, producing potassium hydroxide (KOH) and hydrogen gas (H₂). The reaction is highly exothermic and can even be explosive. Potassium also readily reacts with halogens (like chlorine), forming ionic compounds like potassium chloride (KCl). This capacity to form ionic bonds underpins its essential role in biological systems and industrial applications.
Potassium's Biological Role
Potassium plays a pivotal role in numerous biological functions. Its ions are essential for maintaining the proper fluid balance within cells, nerve impulse transmission, muscle contraction, and enzyme activity. Inadequate potassium levels can lead to various health problems, emphasizing its importance in human biology and health.
Industrial Applications of Potassium
Potassium compounds have numerous industrial uses, including fertilizers (potassium is a macronutrient for plants), glass manufacturing, and the production of various chemicals. Its unique chemical properties make it a valuable component in various industrial processes.
Conclusion: Understanding Potassium's Electron Configuration
The ground state electron configuration of potassium, whether expressed as 1s²2s²2p⁶3s²3p⁶4s¹ or its more concise noble gas notation [Ar]4s¹, is fundamental to understanding its chemical properties and biological significance. Its single valence electron dictates its high reactivity, ease of ionization, and propensity to form +1 ions. This understanding lays the foundation for comprehending potassium's role in biological systems, chemical reactions, and diverse industrial applications. The principles of the Aufbau principle, Hund's rule, and the Pauli exclusion principle provide the framework for predicting and interpreting the electron configuration of not only potassium but also a wide range of other elements, highlighting the fundamental importance of quantum mechanics in chemistry. Further exploration into the intricacies of atomic structure and electron behavior will further illuminate the fascinating world of chemical reactivity and the properties of elements.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 4 And 9
Mar 15, 2025
-
Iodine Is Essential For The Synthesis Of
Mar 15, 2025
-
Is Osmosis High To Low Or Low To High
Mar 15, 2025
-
Concave Mirror And Convex Mirror Difference
Mar 15, 2025
-
Which Is Not A Cranial Bone Of The Skull
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Ground State Electron Configuration For Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
