Give Iupac Names For The Following Structures
News Leon
Apr 06, 2025 · 6 min read
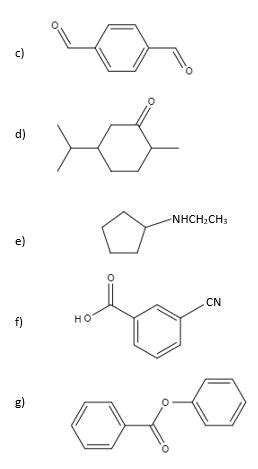
Table of Contents
Giving IUPAC Names to Organic Structures: A Comprehensive Guide
Naming organic compounds might seem daunting at first, but with a systematic approach, it becomes a manageable and even enjoyable skill. The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules to provide a unique and unambiguous name for every organic molecule, regardless of its complexity. This guide will walk you through the process, equipping you to confidently name a wide variety of organic structures. We'll cover alkanes, alkenes, alkynes, alcohols, ketones, aldehydes, carboxylic acids, and more, illustrating each with examples.
Understanding the Basics: Alkane Nomenclature
Before diving into more complex structures, let's establish a foundation with alkanes – saturated hydrocarbons with only single bonds. The naming system hinges on identifying the longest continuous carbon chain, which forms the parent alkane.
Identifying the Parent Chain
Step 1: Find the Longest Carbon Chain. This is crucial. Even if the molecule has branches, you prioritize finding the longest continuous sequence of carbon atoms.
Step 2: Name the Parent Alkane. The number of carbon atoms in the longest chain dictates the parent name. Here are the first ten:
- 1 carbon: Methane
- 2 carbons: Ethane
- 3 carbons: Propane
- 4 carbons: Butane
- 5 carbons: Pentane
- 6 carbons: Hexane
- 7 carbons: Heptane
- 8 carbons: Octane
- 9 carbons: Nonane
- 10 carbons: Decane
Incorporating Substituents
Substituents are groups attached to the parent chain. These are usually alkyl groups (derived from alkanes by removing a hydrogen atom). They are named by removing the "-ane" suffix and adding "-yl". For example, a methyl group (-CH₃) comes from methane, and an ethyl group (-CH₂CH₃) comes from ethane.
Step 3: Number the Carbon Atoms. Number the carbon atoms in the parent chain starting from the end closest to the first substituent. If the substituents are equidistant from both ends, number the chain to give the lowest number to the substituent that appears first alphabetically.
Step 4: Name the Substituents. Identify and name each substituent attached to the parent chain.
Step 5: Combine the Information. The IUPAC name is formed by listing the substituents alphabetically (ignoring prefixes like di- or tri-), followed by the parent alkane name. Use hyphens to separate numbers from words and commas to separate numbers.
Example: Consider a molecule with a longest chain of 4 carbons (butane) and a methyl group on the second carbon. The IUPAC name would be 2-methylbutane.
Alkenes and Alkynes: Incorporating Unsaturation
Alkenes contain carbon-carbon double bonds, while alkynes contain carbon-carbon triple bonds. Their IUPAC names incorporate these features.
Naming Alkenes
Step 1: Find the Longest Chain Containing the Double Bond. This might be shorter than the overall longest chain if the double bond is not part of it.
Step 2: Number the Carbons. Number the carbons to give the double bond the lowest possible number. The position of the double bond is indicated by the number of the first carbon atom involved in the double bond.
Step 3: Name the Alkene. Replace the "-ane" suffix of the corresponding alkane with "-ene".
Example: CH₃CH=CHCH₃ is 2-butene.
Naming Alkynes
Similar to alkenes, alkynes are named by:
Step 1: Find the Longest Chain Containing the Triple Bond.
Step 2: Number the Carbons. Number the carbons to give the triple bond the lowest possible number.
Step 3: Name the Alkyne. Replace the "-ane" suffix with "-yne".
Example: CH₃C≡CCH₃ is 2-butyne.
Functional Groups: Adding Complexity
Functional groups are specific groups of atoms within a molecule that confer characteristic chemical properties. Their presence dictates the suffix of the IUPAC name.
Alcohols (-OH)
Alcohols contain a hydroxyl group (-OH). The naming process is:
Step 1: Identify the Longest Chain Containing the -OH group.
Step 2: Number the Carbons. The carbon bearing the -OH group gets the lowest possible number.
Step 3: Name the Alcohol. Replace the "-e" ending of the alkane with "-ol".
Example: CH₃CH₂OH is ethanol. CH₃CH₂CH₂OH is propan-1-ol. CH₃CH(OH)CH₃ is propan-2-ol.
Ketones (C=O)
Ketones have a carbonyl group (C=O) bonded to two alkyl or aryl groups.
Step 1: Identify the Longest Chain Containing the C=O group.
Step 2: Number the Carbons. The carbonyl carbon receives the lowest possible number.
Step 3: Name the Ketone. Replace the "-e" ending of the alkane with "-one". The position of the carbonyl group is indicated by a number.
Example: CH₃COCH₃ is propan-2-one (also known as acetone).
Aldehydes (CHO)
Aldehydes have a carbonyl group (C=O) at the end of a carbon chain.
Step 1: Identify the Longest Chain Containing the CHO group. The carbonyl carbon is always carbon 1.
Step 2: Name the Aldehyde. Replace the "-e" ending of the alkane with "-al".
Example: CH₃CHO is ethanal.
Carboxylic Acids (COOH)
Carboxylic acids have a carboxyl group (-COOH) at the end of a carbon chain.
Step 1: Identify the Longest Chain Containing the COOH group. The carboxyl carbon is always carbon 1.
Step 2: Name the Carboxylic Acid. Replace the "-e" ending of the alkane with "-oic acid".
Example: CH₃COOH is ethanoic acid (also known as acetic acid).
Dealing with Multiple Substituents and Complex Structures
When multiple substituents are present, the following rules apply:
- Alphabetical Order: List substituents alphabetically, ignoring prefixes like di-, tri-, tetra-.
- Prefixes: Use di-, tri-, tetra- etc. to indicate multiple occurrences of the same substituent.
- Locants: Use numbers to indicate the position of each substituent on the parent chain.
- Hyphens and Commas: Use hyphens to separate numbers from words and commas to separate numbers from each other.
Examples of Complex IUPAC Naming
Let's tackle some more complex examples to solidify our understanding. Remember to always follow the steps meticulously.
Example 1: A molecule with a longest chain of 6 carbons (hexane), two methyl groups on carbons 2 and 4, and an ethyl group on carbon 3.
The name would be 3-ethyl-2,4-dimethylhexane.
Example 2: A molecule with a cyclohexane ring and a methyl group on carbon 1 and a bromine atom on carbon 3.
The name would be 1-methyl-3-bromocyclohexane.
Example 3: A molecule with a benzene ring substituted with a methyl group and a nitro group.
This would be 1-methyl-3-nitrobenzene (or m-nitrotoluene using common naming).
Conclusion: Mastering IUPAC Nomenclature
Mastering IUPAC nomenclature requires practice and attention to detail. However, by systematically following the rules outlined here, you can confidently name a wide array of organic compounds, regardless of their complexity. Remember the importance of identifying the longest chain, assigning numbers correctly, and using the appropriate prefixes and suffixes. With diligent practice, this seemingly complex task will become second nature, enhancing your understanding and appreciation for the world of organic chemistry. Continuous engagement with practice problems and reviewing the core principles will solidify your skillset and enable you to confidently navigate the intricacies of chemical nomenclature.
Latest Posts
Latest Posts
-
The Resistance Of A Wire Depends On
Apr 07, 2025
-
What Is The Relative Charge Of A Neutron
Apr 07, 2025
-
Choose The Two Functions Of The Aug Codon
Apr 07, 2025
-
Why Do Carbon Form Covalent Bond
Apr 07, 2025
-
The Serum Elisa Test Is Based On Interaction Between
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Give Iupac Names For The Following Structures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
