Why Do Carbon Form Covalent Bond
News Leon
Apr 07, 2025 · 6 min read
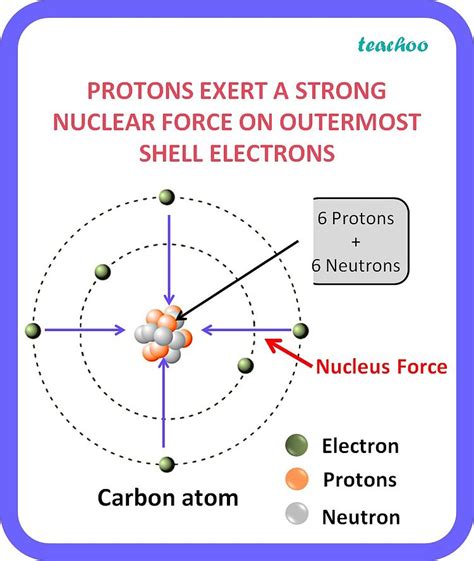
Table of Contents
Why Does Carbon Form Covalent Bonds? Understanding Carbon's Bonding Behavior
Carbon, the backbone of life, is a remarkable element with unique bonding properties. Its ability to form strong, stable covalent bonds is the foundation for the immense diversity of organic molecules found in living organisms and countless synthetic materials. But why does carbon exhibit this preference for covalent bonding? The answer lies in its electronic structure and the energetic stability achieved through shared electron pairs.
Carbon's Electronic Structure: The Key to Covalent Bonding
To understand carbon's bonding behavior, we must first examine its electronic configuration. Carbon has an atomic number of 6, meaning it possesses six electrons. These electrons are distributed in its electron shells as follows: two electrons fill the inner shell (1s²), and four electrons occupy the outer shell (2s²2p²). These four outer electrons are valence electrons, participating directly in chemical bonding.
It's crucial to understand that carbon doesn't readily lose or gain four electrons to achieve a stable noble gas configuration. Losing four electrons to form a C⁴⁺ ion would require an enormous amount of energy, making it highly unfavorable. Similarly, gaining four electrons to form a C⁴⁻ ion would also be incredibly energy-intensive due to the strong electron-electron repulsions within the small carbon atom.
Instead of ionic bonding (the transfer of electrons), carbon achieves stability through covalent bonding, where it shares its four valence electrons with other atoms. This sharing of electrons allows carbon to effectively fill its outer electron shell, achieving a stable octet (eight electrons) similar to that of the noble gases. This stable octet configuration represents a state of minimum energy, making covalent bonding energetically favorable for carbon.
The Versatility of Carbon's Covalent Bonds
The four valence electrons of carbon enable it to form up to four covalent bonds. This capacity for multiple bonding is a defining characteristic of carbon and the reason for the astounding variety of organic compounds. Carbon atoms can bond with:
-
Other carbon atoms: This ability is crucial for the formation of long chains, branched structures, and rings, the foundation of organic chemistry. Carbon's capacity for catenation (the self-linking of atoms of the same element) is unmatched among the elements.
-
Hydrogen atoms: Carbon-hydrogen bonds are exceptionally strong and abundant in organic molecules. These bonds are the basis of hydrocarbons, the simplest organic compounds.
-
Oxygen atoms: Carbon-oxygen bonds are found in many important functional groups, such as alcohols (-OH), ketones (=O), and carboxylic acids (-COOH), influencing the properties and reactivity of organic molecules.
-
Nitrogen atoms: Carbon-nitrogen bonds are prevalent in amines (-NH2), amides (-CONH2), and other crucial nitrogen-containing organic compounds, such as amino acids, the building blocks of proteins.
-
Halogen atoms: Carbon can form bonds with halogens (fluorine, chlorine, bromine, iodine), adding to the diversity of organic molecules and impacting their properties.
-
Sulfur atoms: Carbon-sulfur bonds occur in thiols (-SH) and other sulfur-containing compounds, playing roles in various biological processes.
Types of Covalent Bonds Formed by Carbon
Carbon's ability to form various types of covalent bonds further contributes to the diversity of organic molecules. These include:
-
Single bonds: A single covalent bond involves the sharing of one pair of electrons between two atoms. These bonds are relatively long and weak compared to multiple bonds.
-
Double bonds: A double bond involves the sharing of two pairs of electrons between two atoms. These bonds are shorter and stronger than single bonds. Double bonds often result in restricted rotation around the bond axis, leading to isomerism (molecules with the same atoms but different arrangements).
-
Triple bonds: A triple bond involves the sharing of three pairs of electrons between two atoms. These bonds are the shortest and strongest of the three types, exhibiting even greater rigidity than double bonds. Triple bonds are typically found in alkynes (compounds containing carbon-carbon triple bonds).
-
Resonance structures: In some molecules, electrons are delocalized, meaning they are not confined to a single bond but are shared across multiple atoms. This delocalization leads to resonance structures, contributing to the stability of the molecule. Benzene, a classic example, displays resonance stabilization.
The Significance of Carbon's Covalent Bonding
The unique properties of carbon's covalent bonding have profound implications:
-
Formation of complex molecules: The ability to form long chains, branched structures, and rings leads to the formation of incredibly complex molecules with diverse functionalities. This complexity is essential for the intricate structures and functions of biological molecules like proteins, DNA, and carbohydrates.
-
Diversity of organic compounds: The sheer number of organic compounds is staggering, vastly exceeding the number of inorganic compounds. This vast diversity stems directly from carbon's ability to form multiple bonds with itself and other elements.
-
Isomerism: Carbon's ability to form multiple bonds and branched structures leads to isomerism, meaning molecules with the same formula can have different structures and properties. This isomerism contributes significantly to the complexity and diversity of organic chemistry.
-
Structural stability: Carbon-carbon and carbon-hydrogen bonds are generally strong and stable, resulting in the stability of many organic molecules under various conditions.
-
Functional group chemistry: The various functional groups containing carbon-oxygen, carbon-nitrogen, and other bonds have characteristic reactivities, allowing chemists to design and synthesize countless organic compounds with specific desired properties.
Why Not Other Bonding Types? A Comparative Analysis
While covalent bonding is dominant for carbon, let's briefly consider why other bonding types are less suitable:
-
Ionic bonding: As discussed earlier, the high energy cost associated with carbon either losing or gaining four electrons renders ionic bonding highly unfavorable. The electrostatic forces required to maintain an ionic compound with a highly charged carbon ion would be extremely strong, leading to instability.
-
Metallic bonding: Metallic bonding involves a sea of delocalized electrons shared among many metal atoms. Carbon's electron configuration doesn't lend itself to this type of bonding. While some allotropes of carbon like graphite exhibit some metallic characteristics due to delocalized electrons within the layers, this is not the primary bonding type for carbon.
In summary, carbon's preference for covalent bonding is driven by its electronic structure and the energetic stability achieved through the sharing of electrons. This capacity for diverse covalent bonding is the fundamental reason behind the remarkable diversity and complexity of organic chemistry and the prevalence of carbon-based compounds in living systems. It's the unique combination of its ability to form strong, stable, single, double, and triple bonds with itself and a wide array of other elements that makes carbon the fundamental building block of life and the basis for an enormous range of synthetic materials. Understanding this inherent bonding behavior is key to comprehending the vast and intricate world of organic chemistry and its applications across science, technology, and everyday life.
Latest Posts
Latest Posts
-
Identify The Substrate In The Following Reaction
Apr 09, 2025
-
Which Of The Following Is Not A Characteristic Of Inflammation
Apr 09, 2025
-
Match The Regeneration Capacity Of The Following Tissues
Apr 09, 2025
-
What Is The Resistance Of An Ideal Voltmeter
Apr 09, 2025
-
A Mixed Nerve Is One That Contains Both
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Why Do Carbon Form Covalent Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.