Freezing Point Of Water In Kelvin Scale
News Leon
Mar 17, 2025 · 6 min read
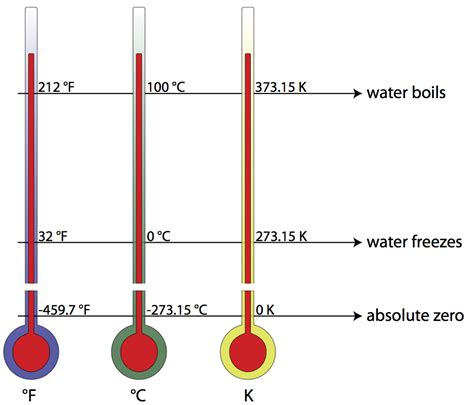
Table of Contents
Freezing Point of Water in Kelvin Scale: A Deep Dive
The freezing point of water is a fundamental concept in science, forming the basis for numerous calculations and applications. While commonly expressed in Celsius (°C) or Fahrenheit (°F), understanding its equivalent in the Kelvin scale (K) is crucial for various scientific disciplines, particularly those involving thermodynamics and cryogenics. This article delves deep into the freezing point of water in Kelvin, exploring its significance, the underlying scientific principles, and its implications across diverse fields.
Understanding the Kelvin Scale
Before we dive into the freezing point, let's establish a solid understanding of the Kelvin scale itself. Unlike Celsius and Fahrenheit, which are relative scales based on the freezing and boiling points of water, the Kelvin scale is an absolute temperature scale. This means its zero point (0 K) represents absolute zero, the theoretically lowest possible temperature where all molecular motion ceases.
This absolute nature of the Kelvin scale makes it the preferred scale in scientific research, particularly in fields like thermodynamics and physics. Many fundamental physical laws and equations are expressed in terms of Kelvin, ensuring consistency and accuracy in calculations. The relationship between Kelvin and Celsius is straightforward:
- K = °C + 273.15
This formula allows for easy conversion between the two scales.
The Significance of Absolute Zero
The concept of absolute zero is pivotal in understanding the Kelvin scale. At absolute zero, the particles of a substance possess minimal energy, and any further reduction in temperature is theoretically impossible. While reaching absolute zero is practically unattainable, scientists constantly strive to achieve temperatures increasingly closer to it, opening up new possibilities in fields like superconductivity and quantum physics.
The Freezing Point of Water in Kelvin
The freezing point of water at standard atmospheric pressure (1 atm) is 0°C. Using the conversion formula mentioned earlier, we can readily determine its Kelvin equivalent:
- K = 0°C + 273.15 = 273.15 K
Therefore, the freezing point of water is 273.15 Kelvin. This value is a cornerstone in various scientific calculations and provides a constant reference point for temperature measurements.
Why is 273.15 K significant?
The significance of 273.15 K extends beyond a simple conversion. This value is fundamentally important because:
-
It defines a crucial phase transition: The transition from liquid water to solid ice at 273.15 K is a precisely defined phase change. This phase transition is crucial for numerous natural processes and industrial applications.
-
It serves as a calibration point: 273.15 K is used as a calibration point for thermometers and other temperature-measuring instruments. Accurate calibration ensures reliable and consistent temperature measurements across different experiments and applications.
-
It's crucial for thermodynamic calculations: Many thermodynamic equations and calculations rely on the absolute temperature scale, with 273.15 K serving as a fundamental reference point.
The Influence of Pressure on the Freezing Point
While 273.15 K is the freezing point at standard atmospheric pressure, it's crucial to acknowledge that pressure can influence the freezing point of water. Increasing pressure lowers the freezing point slightly. This phenomenon is known as pressure melting.
This effect is relatively small at pressures encountered in everyday life but becomes significant under high pressures, such as those encountered in deep ocean trenches or during high-pressure experiments in laboratories. The relationship between pressure and freezing point is described by the Clausius-Clapeyron equation, a complex thermodynamic relationship that governs phase transitions.
Practical Implications of Pressure-Induced Changes
Understanding the pressure dependence of the freezing point of water is crucial in several applications:
-
Glaciology: In studying glaciers and ice sheets, pressure changes with depth significantly impact the melting and refreezing of ice. Pressure-induced melting at the base of glaciers can facilitate glacial flow and contribute to sea-level rise.
-
High-pressure experiments: In scientific research involving high pressures, the precise temperature control becomes critical. Understanding the pressure effect on the freezing point of water ensures accurate experimental conditions.
-
Material science: In material science, particularly in the synthesis and characterization of materials, pressure-induced changes in phase transitions must be accounted for.
Applications of the Freezing Point of Water in Kelvin
The freezing point of water in Kelvin finds applications in a wide range of scientific and engineering disciplines:
1. Cryogenics:
Cryogenics involves the production and application of extremely low temperatures. Precise temperature control, often expressed in Kelvin, is paramount in cryogenic applications, from liquefying gases to superconducting materials research. The freezing point of water provides a benchmark for these low-temperature processes.
2. Food Science and Technology:
In the food industry, understanding the freezing point of water is crucial for processes like freezing food products. The freezing point can vary depending on the solute concentration in the food, and controlling the freezing process is crucial for maintaining food quality and preventing damage to the structure.
3. Meteorology:
Meteorological studies often involve temperature measurements, with the freezing point of water serving as a crucial reference point. Understanding how atmospheric pressure and temperature interact near the freezing point of water is vital for accurate weather forecasting and climate modelling.
4. Environmental Science:
Environmental studies, including hydrology and oceanography, rely heavily on temperature measurements. The freezing point of water is pivotal in understanding processes like ice formation, snow accumulation, and the freezing of water bodies.
5. Chemical Engineering:
Chemical processes often involve phase changes and temperature control. The freezing point of water serves as a reference point in many chemical engineering calculations, particularly in processes involving aqueous solutions or the handling of materials susceptible to freezing.
Beyond the Simple Freezing Point: Supercooling and Other Anomalies
While 273.15 K is the theoretical freezing point, water can sometimes remain liquid below this temperature. This phenomenon is known as supercooling, where water can exist in a metastable liquid state below its normal freezing point due to the absence of nucleation sites for ice crystal formation. However, even slight disturbances can trigger rapid freezing.
Water exhibits several other unusual properties compared to other substances. These include its maximum density at 4°C (277.15 K) and its relatively high heat capacity. These anomalous properties have significant implications for various natural phenomena and technological applications.
Conclusion
The freezing point of water in Kelvin, 273.15 K, is more than just a converted temperature value. It represents a fundamental constant in numerous scientific disciplines, providing a crucial reference point for temperature measurements, calculations, and the understanding of phase transitions. From cryogenics to meteorology, the implications of this value are vast and far-reaching, highlighting its importance in our scientific understanding of the world around us. The further exploration of water’s properties and behavior near its freezing point continues to be a topic of intense scientific inquiry, with potential for significant breakthroughs in diverse fields.
Latest Posts
Latest Posts
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point Of Water In Kelvin Scale . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
