Ethyl Alcohol And Acetic Acid Reaction
News Leon
Mar 21, 2025 · 5 min read
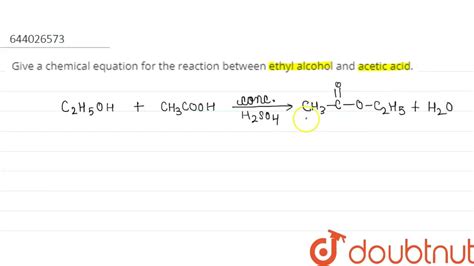
Table of Contents
Ethyl Alcohol and Acetic Acid Reaction: A Deep Dive into Esterification
The reaction between ethyl alcohol (ethanol) and acetic acid (ethanoic acid) is a classic example of esterification, a fundamental organic chemistry reaction with widespread applications. This process, also known as Fischer esterification, produces ethyl acetate, a fragrant ester commonly used as a solvent and in the production of various products. Understanding the mechanism, factors influencing the reaction, and the applications of the resulting ester is crucial for anyone studying organic chemistry or working in related fields. This comprehensive article delves into the intricacies of this reaction, exploring its mechanism, kinetics, and practical applications.
Understanding the Reactants: Ethyl Alcohol and Acetic Acid
Before diving into the reaction itself, let's briefly examine the properties of the reactants: ethyl alcohol and acetic acid.
Ethyl Alcohol (Ethanol)
Ethyl alcohol, or ethanol (CH₃CH₂OH), is a colorless, flammable liquid with a characteristic odor. It's a crucial industrial chemical and a significant component of alcoholic beverages. Its hydroxyl (-OH) group is the key functional group participating in the esterification reaction. Ethanol's polarity and ability to form hydrogen bonds contribute to its solubility in water and other polar solvents.
Acetic Acid (Ethanoic Acid)
Acetic acid, or ethanoic acid (CH₃COOH), is another crucial organic compound, known for its sharp, pungent odor. It's the main component of vinegar and is widely used in various industrial applications, including the production of polymers and pharmaceuticals. The carboxyl group (-COOH), featuring both a carbonyl (C=O) and a hydroxyl group (-OH), is the key functional group involved in the esterification reaction with ethanol. The acidic nature of acetic acid plays a significant role in the reaction mechanism.
The Esterification Reaction: Mechanism and Kinetics
The reaction between ethyl alcohol and acetic acid results in the formation of ethyl acetate and water. This is a reversible reaction, meaning it proceeds in both forward and backward directions.
The Reaction Equation
The balanced chemical equation for the reaction is:
CH₃CH₂OH + CH₃COOH ⇌ CH₃COOCH₂CH₃ + H₂O
Where:
- CH₃CH₂OH represents ethyl alcohol
- CH₃COOH represents acetic acid
- CH₃COOCH₂CH₃ represents ethyl acetate
- H₂O represents water
The Mechanism: A Step-by-Step Approach
The esterification reaction proceeds through an acid-catalyzed mechanism, typically using strong acids like sulfuric acid (H₂SO₄) or hydrochloric acid (HCl) as catalysts. The mechanism involves several steps:
-
Protonation of the Carboxylic Acid: The acid catalyst protonates the carbonyl oxygen of acetic acid, making it more electrophilic. This increases the susceptibility of the carbonyl carbon to nucleophilic attack.
-
Nucleophilic Attack: The oxygen atom of the hydroxyl group in ethanol acts as a nucleophile, attacking the electrophilic carbonyl carbon of the protonated acetic acid. This forms a tetrahedral intermediate.
-
Proton Transfer: A proton transfer occurs within the tetrahedral intermediate, leading to the formation of a neutral molecule with a good leaving group (water).
-
Elimination of Water: The water molecule leaves, resulting in the formation of a protonated ester.
-
Deprotonation: The protonated ester is deprotonated by a water molecule or another base, yielding the final product, ethyl acetate.
Reaction Kinetics: Factors Affecting the Rate
Several factors influence the rate of the esterification reaction:
-
Concentration of Reactants: Increasing the concentration of either ethanol or acetic acid will increase the reaction rate, according to the law of mass action.
-
Acid Catalyst Concentration: The concentration of the acid catalyst significantly affects the reaction rate. A higher concentration of the catalyst leads to a faster reaction.
-
Temperature: Higher temperatures generally accelerate the reaction rate by increasing the kinetic energy of the molecules and the frequency of collisions. However, excessively high temperatures can lead to side reactions.
-
Solvent: The choice of solvent can also influence the reaction rate. Polar solvents often facilitate the reaction, while non-polar solvents may hinder it.
Shifting Equilibrium: Le Chatelier's Principle
As mentioned earlier, the esterification reaction is reversible. To maximize the yield of ethyl acetate, we can apply Le Chatelier's principle, which states that a system at equilibrium will shift to counteract any stress applied to it. Several strategies can be employed:
-
Excess Reactant: Using an excess of one reactant, either ethanol or acetic acid, will shift the equilibrium towards the product side.
-
Water Removal: Removing the water produced during the reaction will also shift the equilibrium to the right, favoring the formation of ethyl acetate. Techniques like azeotropic distillation are commonly used for this purpose.
Applications of Ethyl Acetate
Ethyl acetate is a versatile compound with numerous applications across various industries:
-
Solvent: It's a widely used solvent in various industries, including the pharmaceutical, paint, and coatings industries. Its low toxicity and pleasant odor make it a preferred choice for many applications.
-
Flavor and Fragrance: Ethyl acetate contributes to the characteristic aroma of many fruits and is used in the food and beverage industry as a flavoring agent.
-
Nail Polish Remover: It's a common ingredient in nail polish removers due to its ability to dissolve the film-forming components of nail polish.
-
Cleaning Agent: Its solvent properties also make it suitable for use in various cleaning agents and degreasers.
-
Chemical Intermediate: Ethyl acetate serves as an important chemical intermediate in the synthesis of other chemicals and polymers.
Safety Precautions and Handling
Both ethanol and acetic acid are relatively safe when handled properly, but precautions should always be taken:
-
Ethanol: While relatively low in toxicity compared to other alcohols, ingestion of large quantities can be harmful. It is flammable and should be kept away from open flames.
-
Acetic Acid: Acetic acid is corrosive and can cause skin irritation and burns. It should be handled with appropriate safety equipment, such as gloves and eye protection.
Conclusion: A Versatile Reaction with Wide-Reaching Applications
The esterification reaction between ethyl alcohol and acetic acid is a cornerstone of organic chemistry, offering a fascinating glimpse into reaction mechanisms and equilibrium principles. The resulting ethyl acetate is a versatile compound with numerous applications, highlighting the practical importance of this seemingly simple reaction. By understanding the factors influencing the reaction and the properties of the products, we can appreciate the significance of this reaction in various industrial processes and everyday life. Further research and innovation continue to explore new applications and methods for optimizing this fundamental chemical transformation.
Latest Posts
Latest Posts
-
What Are The Differences Between Plant And Animal
Mar 21, 2025
-
Anything That Occupies Space And Has Mass
Mar 21, 2025
-
Which Statement Is Not True About Dna Replication
Mar 21, 2025
-
What Is The Molar Mass Of Ch3oh
Mar 21, 2025
-
Is It Better To Write Zn2 Or Zn 2
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Ethyl Alcohol And Acetic Acid Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
