Equal To The Number Of Protons
News Leon
Mar 27, 2025 · 6 min read
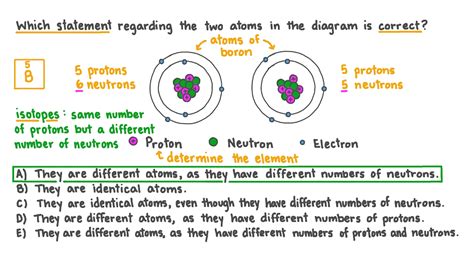
Table of Contents
Atomic Number: Equal to the Number of Protons
The seemingly simple statement, "atomic number is equal to the number of protons," underpins the entire field of chemistry and a significant portion of physics. It's a fundamental concept that unlocks our understanding of the elements, their properties, and how they interact. This article delves deep into the meaning of atomic number, its significance in identifying elements, its relationship with other atomic properties, and its implications across various scientific disciplines.
Understanding Atomic Number: The Defining Characteristic of an Element
The atomic number, often represented by the symbol Z, is a crucial property of an atom. It's not just a number; it's the identity card of an element. Simply put, the atomic number of an element is equal to the number of protons found in the nucleus of a single atom of that element. This number is unique to each element and remains constant for all atoms of that specific element.
Protons: The Heart of the Atom
Protons, along with neutrons, reside in the atom's nucleus, the dense core at the center. Protons carry a positive electrical charge, while neutrons are electrically neutral. The number of protons dictates the element's identity because it directly determines the number of electrons orbiting the nucleus.
Electrons and Chemical Behavior
Electrons, carrying a negative electrical charge, occupy specific energy levels or shells surrounding the nucleus. The number of electrons in the outermost shell, known as the valence electrons, plays a crucial role in determining the element's chemical behavior and its ability to form chemical bonds with other atoms. Since atoms are electrically neutral, the number of electrons in a neutral atom is always equal to the number of protons – hence the significance of the atomic number in understanding electron configuration.
The Periodic Table: Organized by Atomic Number
The Periodic Table of Elements is a visual representation of the elements arranged in increasing order of their atomic numbers. This arrangement isn't arbitrary; it reflects the periodic recurrence of similar chemical properties among elements. Elements with similar electron configurations and, consequently, similar chemical behaviors, are placed in the same column or group.
Groups and Periods: Trends in Properties
The vertical columns are called groups or families, representing elements with similar outer electron configurations and thus similar chemical reactivity. For instance, Group 18 (noble gases) are exceptionally unreactive due to their filled outer electron shells. The horizontal rows are called periods, representing elements with the same number of electron shells. Moving across a period, the atomic number increases, and the properties of the elements change progressively.
Predicting Properties Based on Atomic Number
The arrangement of the periodic table allows scientists to predict the properties of elements based solely on their atomic number. By knowing the atomic number, we can infer the number of electrons, the general electron configuration, and, to a significant extent, the chemical behavior of the element. This predictive power is invaluable in fields like materials science and chemical engineering.
Isotopes: Same Atomic Number, Different Mass Number
While the atomic number defines the element, atoms of the same element can have different numbers of neutrons. These variations are called isotopes. Isotopes of the same element have the same atomic number (same number of protons) but different mass numbers (the sum of protons and neutrons).
Mass Number and Isotopic Abundance
The mass number is crucial in determining the atomic mass of an element, which is a weighted average of the masses of its isotopes, taking into account their natural abundance. Some isotopes are stable, while others are radioactive, undergoing radioactive decay. The study of isotopes and their radioactive properties has wide-ranging applications in various fields, including carbon dating, medical imaging, and nuclear energy.
Atomic Number and Nuclear Reactions
The atomic number plays a pivotal role in understanding nuclear reactions. Nuclear reactions involve changes in the nucleus of an atom, which can alter the number of protons and neutrons. These reactions can lead to the formation of new elements or isotopes.
Nuclear Fission and Fusion
In nuclear fission, a heavy nucleus splits into smaller nuclei, often releasing a tremendous amount of energy. The atomic number of the resulting nuclei differs from that of the original nucleus. In nuclear fusion, lighter nuclei combine to form a heavier nucleus, also releasing substantial energy. Again, the atomic number changes during the process.
Radioactive Decay
Radioactive decay involves the spontaneous transformation of an unstable nucleus into a more stable one, often involving the emission of particles or energy. Alpha decay, beta decay, and gamma decay are common types of radioactive decay, each affecting the atomic number in a different way. Alpha decay reduces the atomic number by 2, while beta decay increases it by 1. Gamma decay doesn't alter the atomic number.
Beyond the Basics: Applications of Atomic Number
The concept of atomic number is far from being merely an academic exercise; it's a cornerstone of numerous scientific and technological advancements:
Spectroscopy: Identifying Elements Through Light
Spectroscopy analyzes the interaction of light with matter. Each element emits or absorbs light at specific wavelengths, creating a unique spectral fingerprint. By analyzing these spectral lines, scientists can identify the elements present in a sample, based on their atomic number and electron configuration. This technique is crucial in astronomy, material analysis, and environmental monitoring.
Nuclear Medicine: Utilizing Isotopes for Diagnosis and Treatment
Radioactive isotopes, with their specific atomic numbers and decay properties, are widely used in medical imaging and therapy. Techniques like PET (Positron Emission Tomography) and SPECT (Single-Photon Emission Computed Tomography) utilize radioactive tracers to diagnose diseases and monitor treatment effectiveness. Radiotherapy also employs radioactive isotopes to target and destroy cancerous cells.
Materials Science: Designing Materials with Specific Properties
Understanding atomic number is essential for materials science. By controlling the composition and arrangement of atoms, with specific atomic numbers and properties, scientists can design materials with desirable characteristics, such as strength, conductivity, or reactivity. This has led to advancements in various industries, including aerospace, electronics, and construction.
Chemical Reactions and Bonding
Atomic number plays a decisive role in determining how atoms interact and form chemical bonds. The number of valence electrons, directly related to the atomic number, dictates an element's bonding capacity and the type of bonds it forms (ionic, covalent, metallic). Understanding bonding is crucial to comprehend the properties of molecules and materials.
Nuclear Physics and Energy Production
The study of atomic nuclei, and thus atomic numbers, is critical in nuclear physics. It's fundamental to understanding nuclear fission and fusion reactions, which are employed in nuclear power plants and nuclear weapons. It also underlies our understanding of radioactive decay and its applications.
Conclusion: The Foundation of Chemistry and Physics
The atomic number, equal to the number of protons in an atom's nucleus, is far more than just a numerical identifier. It's the fundamental property that defines an element, dictates its chemical behavior, and underpins our understanding of the structure of matter and its interactions. Its significance extends far beyond theoretical chemistry and physics; it's a cornerstone of numerous scientific and technological advancements, shaping our world in countless ways. From the development of life-saving medical treatments to the creation of high-performance materials, the knowledge of atomic number remains a critical component of scientific progress. The simple statement – atomic number equals the number of protons – truly holds the key to unlocking the complexities of the universe at its most fundamental level.
Latest Posts
Latest Posts
-
Combine Terms 12a 26b 4b 16a
Mar 30, 2025
-
What Is The Difference Between Obligate And Facultative
Mar 30, 2025
-
What Is The Molecular Mass Of Ch3cooh
Mar 30, 2025
-
How Many Hours Is In 9 Days
Mar 30, 2025
-
The Si Unit Of Power Is
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Equal To The Number Of Protons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
