Electronic Configuration Of Cr And Cu
News Leon
Mar 16, 2025 · 5 min read
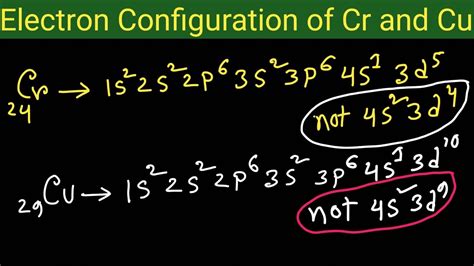
Table of Contents
The Anomalous Electronic Configurations of Chromium (Cr) and Copper (Cu): A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While generally predictable, certain elements defy the standard Aufbau principle, showcasing exceptions that deepen our understanding of atomic interactions. Two notable examples are chromium (Cr) and copper (Cu), which exhibit anomalous electronic configurations. This article delves into the intricacies of their electronic configurations, exploring the reasons behind these exceptions and their implications.
Understanding Electronic Configuration
Before exploring the anomalies, let's review the basics. Electronic configuration describes the arrangement of electrons within the electron shells and subshells of an atom. The Aufbau principle, Hund's rule, and the Pauli exclusion principle govern this arrangement. The Aufbau principle states that electrons fill orbitals in order of increasing energy, starting with the lowest energy level. Hund's rule dictates that electrons fill orbitals individually before pairing up, maximizing spin multiplicity. The Pauli exclusion principle limits each orbital to a maximum of two electrons with opposite spins.
Based on these principles, we'd expect the electronic configuration of chromium (atomic number 24) to be 1s²2s²2p⁶3s²3p⁶4s²3d⁴, and copper (atomic number 29) to be 1s²2s²2p⁶3s²3p⁶4s²3d⁹. However, experimental evidence reveals a different story.
The Anomalous Configuration of Chromium (Cr)
The actual electronic configuration of chromium is 1s²2s²2p⁶3s²3p⁶3d⁵4s¹. Notice the seemingly "out-of-order" arrangement – one electron from the 4s orbital has moved to the 3d orbital, resulting in a half-filled d subshell.
Why the Anomaly?
This anomaly is explained by considering the stability associated with half-filled and completely filled subshells. A half-filled d subshell (five electrons with parallel spins) exhibits extra stability due to:
-
Exchange Energy: Electrons with parallel spins in degenerate orbitals (orbitals with the same energy) experience a repulsive force that lowers the overall energy of the system. This exchange energy is maximized in a half-filled subshell.
-
Symmetrical Electron Distribution: A half-filled d subshell leads to a symmetrical distribution of electron density, contributing to increased stability.
By transferring one electron from the 4s orbital to the 3d orbital, chromium achieves a more stable half-filled 3d subshell, despite the slightly higher energy of the 3d orbitals. This increased stability outweighs the energy cost of the electron promotion.
The Anomalous Configuration of Copper (Cu)
Similar to chromium, copper (Cu) displays an anomalous electronic configuration. Instead of the expected 1s²2s²2p⁶3s²3p⁶4s²3d⁹, its actual configuration is 1s²2s²2p⁶3s²3p⁶3d¹⁰4s¹. This configuration features a completely filled 3d subshell and a singly occupied 4s orbital.
Why the Anomaly?
The reason behind copper's anomalous configuration is also linked to the enhanced stability of a completely filled subshell. A completely filled d subshell (ten electrons) offers significant stability due to:
-
Maximum Exchange Energy: Although exchange energy is maximized with a half-filled subshell, a completely filled subshell also exhibits substantial exchange energy.
-
Increased Shielding Effect: The ten electrons in the filled d subshell effectively shield the outer electrons from the nuclear charge, lowering the overall energy of the atom.
-
Spherical Symmetry: A completely filled d subshell contributes to a more spherical distribution of electron density, further enhancing stability.
Moving one electron from the 4s orbital to complete the 3d subshell results in a more stable electronic configuration for copper, even though the 4s orbital is slightly lower in energy. The gain in stability from the completely filled d subshell supersedes the energy difference.
Implications of Anomalous Configurations
These anomalous configurations have implications for various properties of chromium and copper:
-
Magnetic Properties: The half-filled d subshell in chromium makes it paramagnetic, meaning it is weakly attracted to magnetic fields. The completely filled d subshell in copper, however, renders it diamagnetic, meaning it is not attracted to magnetic fields.
-
Chemical Reactivity: The electronic configurations influence the chemical reactivity of these elements. The relatively stable configurations affect their oxidation states and their tendency to form complexes.
-
Spectroscopic Properties: The electronic configurations directly impact the absorption and emission spectra of these elements. The transitions between energy levels determine the wavelengths of light absorbed or emitted.
-
Metallic Bonding: The arrangement of electrons influences the strength of metallic bonds, impacting properties like melting point and electrical conductivity.
Further Exploration: Beyond Cr and Cu
While chromium and copper are the most prominent examples, other transition metal elements also display slightly irregular electronic configurations. These deviations from the standard Aufbau principle are generally attributed to the relatively small energy difference between the (n-1)d and ns orbitals, leading to subtle energy minimisation strategies. The balance between exchange energy, shielding effects, and orbital energy differences determines the final electronic configuration. Further research into these anomalies requires considering relativistic effects, especially for heavier elements.
Conclusion: The Importance of Exceptions
The anomalous electronic configurations of chromium and copper highlight the limitations of simple rules and the complexities inherent in atomic structure. While the Aufbau principle provides a valuable framework for understanding electronic configurations, it's essential to recognize exceptions and their underlying causes. These exceptions demonstrate the importance of considering factors like exchange energy and the stability associated with half-filled and completely filled subshells in determining the actual electron arrangement. Understanding these anomalies offers a more nuanced and accurate depiction of atomic behavior and provides a deeper appreciation for the intricate dance of electrons within the atom. The study of these exceptions is crucial to comprehending the unique properties and reactivity of transition metals and their crucial roles in various scientific fields and industrial applications. Their behavior emphasizes the need to move beyond simple rules and embrace the complex reality of atomic structure and electron interactions.
Latest Posts
Latest Posts
-
Burning Of Candle Is Chemical Change
Mar 17, 2025
-
An Earth Satellite Moves In A Circular Orbit
Mar 17, 2025
-
What Is The Value Of K In Physics
Mar 17, 2025
-
The Study Of Tissues With A Microscope Is Called
Mar 17, 2025
-
The Male Gamete Is Called The
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Electronic Configuration Of Cr And Cu . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
