Each Hemoglobin Molecule Can Carry How Many Oxygen Molecules
News Leon
Mar 31, 2025 · 5 min read
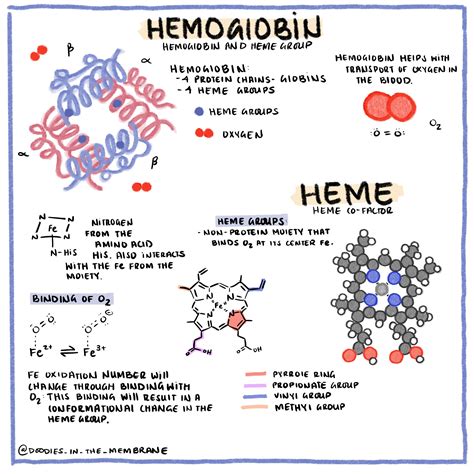
Table of Contents
Each Hemoglobin Molecule Can Carry How Many Oxygen Molecules?
Understanding the oxygen-carrying capacity of hemoglobin is fundamental to comprehending respiration and oxygen transport in the body. This seemingly simple question – how many oxygen molecules can a single hemoglobin molecule carry? – opens the door to a fascinating exploration of molecular biology, physiology, and the intricate mechanisms that sustain life. Let's delve into the details.
The Structure of Hemoglobin: A Marvel of Molecular Engineering
Hemoglobin, a protein found in red blood cells (erythrocytes), is the primary oxygen transporter in vertebrates. Its remarkable ability to bind and release oxygen efficiently is crucial for delivering oxygen to tissues and removing carbon dioxide. The very structure of hemoglobin is designed for this purpose.
Quaternary Structure: The Cooperative Binding Powerhouse
Hemoglobin's quaternary structure is tetrameric, meaning it consists of four subunits arranged together. In adult humans (HbA), these subunits are two alpha (α) and two beta (β) globin chains. Each globin chain is intricately folded and contains a heme group.
Heme: The Oxygen-Binding Site
The heme group is the key player in oxygen binding. It's a porphyrin ring containing a ferrous iron (Fe²⁺) ion at its center. This iron ion is precisely positioned to reversibly bind one oxygen molecule (O₂).
The Magic Number: Four Oxygen Molecules Per Hemoglobin
Given that each hemoglobin molecule possesses four heme groups (one in each globin subunit), the answer is clear: a single hemoglobin molecule can carry four oxygen molecules.
Cooperative Binding: Enhancing Efficiency
The binding of oxygen to hemoglobin isn't simply a matter of four independent events. The binding of one oxygen molecule to a heme group induces a conformational change in the entire hemoglobin molecule. This change makes it easier for subsequent oxygen molecules to bind to the remaining heme groups. This phenomenon is known as cooperative binding, and it significantly enhances the efficiency of oxygen uptake in the lungs, where oxygen partial pressure is high.
The Sigmoidal Oxygen-Hemoglobin Dissociation Curve
The cooperative binding is graphically represented by the sigmoidal (S-shaped) oxygen-hemoglobin dissociation curve. The curve's shape illustrates how hemoglobin's affinity for oxygen changes depending on the oxygen partial pressure. At high oxygen partial pressures (like in the lungs), hemoglobin readily binds oxygen. At lower oxygen partial pressures (like in tissues), hemoglobin releases oxygen readily. This crucial feature ensures efficient oxygen delivery where it's most needed.
Factors Affecting Oxygen Binding: More Than Just Oxygen Partial Pressure
Several other factors influence the binding of oxygen to hemoglobin, affecting the position of the oxygen-hemoglobin dissociation curve. Understanding these factors provides a more complete picture of oxygen transport.
pH: The Bohr Effect
Changes in pH significantly affect oxygen binding. A decrease in pH (increased acidity), such as that occurring in actively metabolizing tissues, reduces hemoglobin's affinity for oxygen. This effect, known as the Bohr effect, facilitates the release of oxygen to tissues that need it most.
Temperature: The Influence of Heat
Higher temperatures also decrease hemoglobin's affinity for oxygen. This is particularly relevant during physical exertion, where increased metabolic activity generates heat. The reduced affinity helps ensure adequate oxygen supply to working muscles.
2,3-Bisphosphoglycerate (2,3-BPG): A Regulatory Molecule
2,3-BPG, a molecule present in red blood cells, binds to hemoglobin and reduces its oxygen affinity. This helps to regulate oxygen release in tissues, particularly in situations of low oxygen availability.
Carbon Dioxide: A Competitive Inhibitor
Carbon dioxide also plays a role. It can directly bind to hemoglobin, reducing oxygen affinity, and it contributes to the decrease in pH, further facilitating oxygen unloading.
Hemoglobin Variants and Their Impact on Oxygen Transport
While HbA is the most common hemoglobin type in adults, several other hemoglobin variants exist. These variants often result from mutations in the globin genes and can significantly affect oxygen transport capacity.
Sickle Cell Anemia: A Devastating Example
Sickle cell anemia, a genetic disorder, exemplifies the impact of a hemoglobin variant. A single amino acid substitution in the beta-globin chain leads to the formation of abnormal hemoglobin (HbS). HbS polymerizes under low oxygen conditions, causing red blood cells to become sickle-shaped, leading to vaso-occlusion and various health complications.
Thalassemia: Imbalance in Globin Chain Synthesis
Thalassemias are a group of inherited blood disorders characterized by reduced or absent synthesis of one or more globin chains. This imbalance affects the formation of functional hemoglobin tetramers and impacts oxygen-carrying capacity.
Beyond Oxygen: Other Roles of Hemoglobin
While oxygen transport is hemoglobin's primary function, it also plays a vital role in carbon dioxide transport. Hemoglobin can bind to carbon dioxide, although not directly at the heme group. This binding facilitates the removal of carbon dioxide from tissues and its transport to the lungs for exhalation.
Conclusion: A Symphony of Molecular Interactions
The answer to "how many oxygen molecules can a single hemoglobin molecule carry?" is definitively four. However, the simplicity of this answer belies the complexity and elegance of the molecular mechanisms involved in oxygen transport. Cooperative binding, the Bohr effect, the influence of temperature, 2,3-BPG, and carbon dioxide all contribute to a finely tuned system that ensures efficient oxygen delivery to tissues throughout the body. Furthermore, the existence of hemoglobin variants underscores the sensitivity of this system to even minor genetic alterations and highlights the vital role of proper hemoglobin function in maintaining health. Understanding these intricacies provides a deeper appreciation of the biological marvel that sustains life.
Latest Posts
Latest Posts
-
For Which Value Of X Is Abcd A Kite
Apr 01, 2025
-
64 To The Power Of 1 2
Apr 01, 2025
-
On The Galapagos Islands Charles Darwin Observed
Apr 01, 2025
-
A Population Is Composed Of Individuals Of
Apr 01, 2025
-
Suppose That An Electric Charge Is Produced
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Each Hemoglobin Molecule Can Carry How Many Oxygen Molecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
