Draw A Mechanism For This Reaction. Interactive 3d Display Mode
News Leon
Mar 19, 2025 · 5 min read
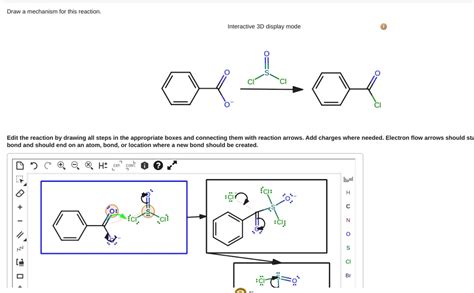
Table of Contents
Draw a Mechanism for This Reaction: Interactive 3D Display Mode
The ability to visualize and interact with chemical reaction mechanisms in 3D is revolutionizing the way we teach and learn organic chemistry. Gone are the days of static 2D diagrams struggling to convey the three-dimensional nature of molecules and their transformations. This article delves into the power of interactive 3D displays in understanding reaction mechanisms, focusing on how to draw and interpret them effectively, particularly focusing on the crucial aspect of visualizing transition states and intermediate structures.
Understanding Reaction Mechanisms: Beyond the Equation
A chemical equation simply shows the starting materials and the products of a reaction. It doesn't explain how the transformation occurs. A reaction mechanism, however, provides a detailed, step-by-step description of this process, including the movement of electrons, the formation and breaking of bonds, and the structures of any intermediates or transition states involved. Visualizing these steps is crucial for a deep understanding.
The Importance of 3D Visualization
Many organic reactions involve stereochemistry – the three-dimensional arrangement of atoms in a molecule. This stereochemistry can significantly influence the outcome of the reaction. A 2D drawing often fails to accurately represent this three-dimensional aspect, leading to confusion and a lack of complete understanding. Interactive 3D models, on the other hand, offer a powerful tool to overcome this limitation. They allow students and researchers to rotate molecules, examine bond angles and lengths, and visualize the changes in molecular geometry throughout the reaction.
Drawing a Mechanism: A Step-by-Step Approach
Let's consider a specific example – an SN1 reaction – to illustrate how to draw a mechanism using interactive 3D visualization tools. Imagine the solvolysis of tert-butyl bromide in water:
(CH3)3CBr + H2O → (CH3)3COH + HBr
This seemingly simple reaction involves several crucial steps, each of which benefits from a 3D representation.
Step 1: The Leaving Group Departure (Ionization)
The first step in the SN1 mechanism is the ionization of the alkyl halide. The C-Br bond breaks heterolytically, with the bromine atom taking both electrons from the bond, forming a bromide ion (Br⁻). This leaves behind a carbocation intermediate – a carbon atom with only three bonds and a positive charge. In a 3D model, we can clearly see the planar geometry of the carbocation, a key feature of this intermediate. The interactive nature allows us to examine the changes in bond angles and lengths as the bond breaks.
Visualizing this in 3D: The model would show the C-Br bond lengthening and eventually breaking, with the bromine atom becoming negatively charged and moving away from the carbon atom. The remaining carbon atom would be shown with three bonds and a positive charge, clearly displaying its planar geometry.
Step 2: Nucleophilic Attack
Water, acting as a nucleophile (an electron-rich species), attacks the carbocation. One of the lone pairs on the oxygen atom of the water molecule forms a bond with the positively charged carbon atom. This step also involves a significant change in geometry around the carbon atom.
Visualizing this in 3D: The 3D model would dynamically show the water molecule approaching the planar carbocation. The formation of the new C-O bond would be clearly visible, and the change from planar to tetrahedral geometry around the carbon atom would be apparent.
Step 3: Proton Transfer
The resulting intermediate is a protonated alcohol. A water molecule then acts as a base, abstracting a proton from the oxygen atom, forming the final alcohol product ((CH3)3COH) and a hydronium ion (H3O⁺).
Visualizing this in 3D: The model would display the interaction between the protonated alcohol and a water molecule. The transfer of the proton would be shown, highlighting the change in bonding and charge distribution.
Interactive 3D Display Modes and Software
Several software packages facilitate the creation and manipulation of 3D molecular models. These include:
- ChemDraw: A widely used chemical drawing software capable of generating 3D structures.
- Avogadro: A free, open-source molecular editor and visualization tool, allowing for interactive 3D modelling.
- Jmol: A Java-based molecular viewer that enables interactive visualization of 3D structures.
- PyMOL: A powerful molecular visualization system with advanced features.
- Spartan: A commercially available suite of computational chemistry programs with impressive visualization capabilities.
These programs offer various functionalities:
- Rotation and Zoom: Allows for exploring the molecule from different perspectives.
- Bond Angle and Length Measurements: Provides quantitative information about the molecular structure.
- Animation: Shows the dynamic changes during the reaction mechanism.
- Electrostatic Potential Maps: Visualizes electron distribution within the molecule.
- Energy Calculations: Enables the estimation of energy changes during the reaction.
Advanced Concepts and Applications
The application of interactive 3D visualization extends beyond simple reaction mechanisms. It can be used to:
- Visualize complex reactions: Multi-step reactions involving numerous intermediates and transition states can be effectively presented and analyzed in 3D.
- Study conformational changes: Conformational changes in molecules often play a crucial role in their reactivity. 3D models can aid in understanding these changes.
- Investigate transition states: Although transition states are high-energy species and cannot be directly observed, their geometries can be predicted using computational methods and visualized using 3D models. This helps understand the reaction pathway.
- Predict reaction outcomes: By modeling the reaction mechanism, one can potentially predict the stereochemistry of the products and the overall reaction yield.
Limitations and Considerations
While interactive 3D visualization offers numerous advantages, some limitations should be acknowledged:
- Computational resources: Some advanced visualization techniques, particularly those involving molecular dynamics simulations, require significant computational resources.
- Software complexity: Mastery of some of the software packages requires time and effort.
- Oversimplification: Models are often simplifications of reality, neglecting certain factors that may influence the actual reaction pathway.
Conclusion: A New Era in Chemical Education and Research
Interactive 3D display modes are transforming our understanding of chemical reaction mechanisms. They provide a more intuitive and engaging learning experience and are indispensable tools in chemical research. By allowing users to actively explore the three-dimensional aspects of molecular transformations, these tools foster deeper understanding, facilitate better problem-solving, and lead to more effective teaching and research. As software advances and computational power increases, the possibilities for interactive 3D visualization in chemistry are only set to expand, paving the way for a new era in chemical education and research. The ability to manipulate and visualize reaction mechanisms in 3D is no longer a luxury, but a necessity for anyone seeking a comprehensive grasp of chemical reactivity.
Latest Posts
Latest Posts
-
How Much Seconds Are In 1 Hour
Mar 19, 2025
-
4 Protons 5 Neutrons 4 Electrons
Mar 19, 2025
-
When You Say The Opposite Of What You Mean
Mar 19, 2025
-
The Apc Can Be Defined As The Fraction Of A
Mar 19, 2025
-
You Throw Away The Outside And Cook The Inside
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Draw A Mechanism For This Reaction. Interactive 3d Display Mode . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
