Does A Gas Have A Definite Volume
News Leon
Mar 14, 2025 · 6 min read
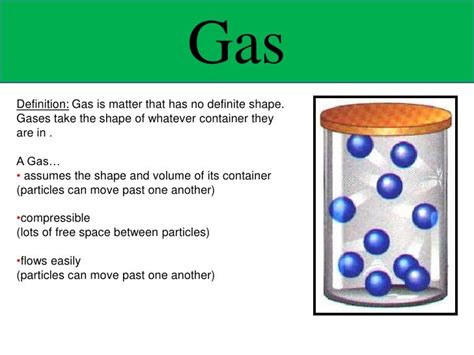
Table of Contents
Does a Gas Have a Definite Volume? Understanding the Nature of Gases
The question of whether a gas has a definite volume is a fundamental concept in chemistry and physics. The short answer is no, a gas does not have a definite volume. Unlike solids and liquids, which maintain a relatively constant shape and volume, gases are highly compressible and readily expand or contract to fill their container. This characteristic stems from the unique properties of gas molecules and their interactions. Let's delve deeper into this fascinating topic, exploring the behavior of gases, the factors influencing their volume, and the implications of this understanding in various scientific fields.
Understanding the Kinetic Molecular Theory of Gases
To understand why gases don't have a definite volume, we must first understand the Kinetic Molecular Theory of Gases (KMTG). This theory provides a microscopic model to explain the macroscopic behavior of gases. The key postulates of KMTG are:
- Gases are composed of tiny particles (atoms or molecules) that are in constant, random motion. This motion is responsible for the pressure exerted by the gas.
- The volume of the gas particles themselves is negligible compared to the total volume occupied by the gas. This means that the particles are far apart from each other.
- There are no attractive or repulsive forces between gas particles. This simplifies the model, although real gases exhibit some intermolecular forces, particularly at high pressures and low temperatures.
- The collisions between gas particles and the walls of the container are perfectly elastic. This means that kinetic energy is conserved during collisions.
- The average kinetic energy of the gas particles is directly proportional to the absolute temperature of the gas. This implies that higher temperatures lead to faster-moving particles and higher pressure.
These postulates directly explain why gases don't possess a definite volume. Because the gas particles are widely dispersed and have negligible volume compared to the container, they readily adapt to the available space. The particles are not bound to specific locations like those in solids or liquids, allowing them to expand to fill any container they are placed in.
Factors Affecting the Volume of a Gas
While a gas doesn't have an inherent, fixed volume, its volume is certainly not arbitrary. Several factors influence the volume a gas occupies:
1. Pressure (P):
Pressure is the force exerted per unit area by the gas particles colliding with the walls of the container. Increasing the pressure on a gas will decrease its volume, as the particles are compressed into a smaller space. Conversely, decreasing the pressure will allow the gas to expand and occupy a larger volume. This relationship is inversely proportional and is described by Boyle's Law: P₁V₁ = P₂V₂, where P and V represent pressure and volume, respectively, and the subscripts 1 and 2 represent initial and final states.
2. Temperature (T):
Temperature is a measure of the average kinetic energy of the gas particles. Increasing the temperature increases the kinetic energy of the particles, causing them to move faster and collide more forcefully with the container walls. This leads to an increase in volume if the pressure is kept constant. Decreasing the temperature has the opposite effect. This relationship is directly proportional and is described by Charles's Law: V₁/T₁ = V₂/T₂, where T represents the absolute temperature (in Kelvin).
3. Number of Moles (n):
The number of moles of gas represents the amount of gas present. Increasing the number of moles increases the number of gas particles, leading to more frequent collisions and a larger volume if pressure and temperature remain constant. Decreasing the number of moles has the opposite effect. This relationship is directly proportional and is embodied in Avogadro's Law: V₁/n₁ = V₂/n₂.
4. The Ideal Gas Law: Combining the Factors
The Ideal Gas Law combines the relationships described above into a single equation: PV = nRT, where:
- P = Pressure
- V = Volume
- n = Number of moles
- R = Ideal gas constant (a proportionality constant)
- T = Temperature (in Kelvin)
This equation accurately predicts the behavior of many gases under moderate conditions. However, it's important to remember that the Ideal Gas Law is a simplification and doesn't perfectly account for the behavior of real gases, especially at high pressures or low temperatures where intermolecular forces become significant.
Real Gases vs. Ideal Gases
The Ideal Gas Law assumes that gas particles have negligible volume and no intermolecular forces. While convenient for many calculations, this is not entirely accurate for real gases. Real gases exhibit deviations from ideal behavior, particularly:
- At high pressures: The volume occupied by the gas particles themselves becomes significant compared to the total volume, leading to a smaller volume than predicted by the Ideal Gas Law.
- At low temperatures: Intermolecular forces become more significant, causing the particles to attract each other and reducing the volume compared to the ideal prediction.
Equations like the van der Waals equation attempt to account for these deviations by introducing correction factors for both intermolecular forces and particle volume. However, even these more complex equations are approximations, and the behavior of real gases can be quite intricate and dependent on specific molecular properties.
Applications of Understanding Gas Volume
The understanding of gas volume and its dependence on pressure, temperature, and the number of moles has crucial applications across various fields:
1. Meteorology:
Weather forecasting relies heavily on understanding gas behavior. Changes in atmospheric pressure, temperature, and humidity (amount of water vapor) directly affect the volume of air masses, influencing weather patterns like wind, storms, and precipitation.
2. Engineering:
Engineers use the principles of gas behavior to design and optimize systems involving gases, such as internal combustion engines, compressors, and gas pipelines. Accurate predictions of gas volume are essential for ensuring efficiency and safety.
3. Chemistry:
In chemistry, understanding gas volume is fundamental to stoichiometric calculations, reaction rate determination, and gas chromatography. The ideal gas law and related concepts are essential tools in many chemical experiments and analyses.
4. Medicine:
In medical applications, understanding gas behavior is important in areas such as respiratory therapy, anesthesia, and the study of gas exchange in the lungs. Accurate measurement and control of gas volumes are crucial for patient safety and effective treatment.
Conclusion: The Indefinite Nature of Gas Volume
In summary, a gas does not have a definite volume. Its volume is highly dependent on pressure, temperature, and the amount of gas present. The Kinetic Molecular Theory provides a microscopic explanation for this behavior, highlighting the constant, random motion of gas particles and their negligible volume compared to the container. While the Ideal Gas Law offers a useful simplification, real gases exhibit deviations from ideal behavior, particularly at high pressures and low temperatures. Understanding these principles is crucial in diverse fields, from weather forecasting to chemical engineering and medical applications, highlighting the far-reaching implications of this fundamental concept. The ability to accurately predict and control gas volume remains a cornerstone of many scientific and technological advancements.
Latest Posts
Latest Posts
-
Reactions Which Do Not Continue To Completion Are Called Reactions
Mar 14, 2025
-
How Many Minutes Is Five Hours
Mar 14, 2025
-
Flowering Plants Are Also Known As
Mar 14, 2025
-
Advantages And Disadvantages Of Proprietary Software
Mar 14, 2025
-
Which Of The Following Has The Shortest Wavelength
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Does A Gas Have A Definite Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
