Do Gases Have A Fixed Volume
News Leon
Mar 15, 2025 · 6 min read
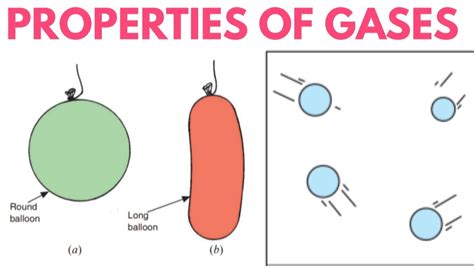
Table of Contents
Do Gases Have a Fixed Volume? Exploring the Properties of Gases
The question of whether gases possess a fixed volume is a fundamental concept in chemistry and physics. The short answer is no, gases do not have a fixed volume. Unlike solids and liquids, which maintain a relatively constant volume regardless of their container, gases are highly compressible and readily adapt to the shape and volume of their enclosure. Understanding this characteristic requires delving into the kinetic molecular theory of gases and exploring the factors that influence gas behavior.
Understanding the Kinetic Molecular Theory of Gases
The kinetic molecular theory (KMT) provides a microscopic explanation for the macroscopic properties of gases. This theory postulates several key assumptions:
-
Gases are composed of tiny particles (atoms or molecules) that are in constant, random motion. These particles are constantly colliding with each other and with the walls of their container.
-
The volume of these particles is negligible compared to the total volume of the gas. This means the spaces between gas particles are vast in comparison to the size of the particles themselves.
-
The attractive and repulsive forces between gas particles are negligible. This assumption holds true for ideal gases, but real gases deviate from ideality at high pressures and low temperatures where intermolecular forces become significant.
-
Collisions between gas particles and the container walls are perfectly elastic. This means no kinetic energy is lost during collisions.
-
The average kinetic energy of gas particles is directly proportional to the absolute temperature (in Kelvin). Higher temperatures result in faster-moving particles.
These assumptions explain why gases expand to fill their containers. The constant, random motion of gas particles causes them to spread out and occupy all available space. There's no inherent "volume" the gas "wants" to occupy; it simply adapts to the available space.
The Implications of Negligible Interparticle Forces
The assumption of negligible interparticle forces is crucial in understanding the lack of fixed volume in gases. Unlike liquids and solids where strong intermolecular forces hold molecules relatively close together, the weak forces in gases allow the particles to move freely and independently. This freedom of movement allows the gas to expand or compress easily. Adding more gas to a container increases the number of particles and their collisions with the walls, thus increasing pressure, but it doesn't inherently change the volume of each individual particle.
Factors Affecting Gas Volume: Pressure, Temperature, and Quantity
While gases don't possess a fixed volume, their volume is definitely influenced by several factors:
1. Pressure (P)
Pressure is the force exerted by gas particles per unit area on the walls of their container. Increasing the pressure on a gas forces the particles closer together, thus decreasing the volume. Conversely, reducing the pressure allows the gas to expand, increasing its volume. This relationship is inversely proportional, as described by Boyle's Law: P₁V₁ = P₂V₂ (at constant temperature and amount of gas).
2. Temperature (T)
Temperature is a measure of the average kinetic energy of gas particles. Increasing the temperature increases the kinetic energy, causing particles to move faster and collide more forcefully with the container walls. This leads to an increase in volume if the pressure is kept constant. Conversely, decreasing the temperature slows down the particles, resulting in a decrease in volume. This relationship is directly proportional, as described by Charles's Law: V₁/T₁ = V₂/T₂ (at constant pressure and amount of gas).
3. Amount of Gas (n)
The amount of gas, usually expressed in moles (n), directly influences the volume. Increasing the number of gas particles increases the number of collisions with the container walls, leading to an increase in volume if the pressure and temperature are kept constant. This relationship is directly proportional, as described by Avogadro's Law: V₁/n₁ = V₂/n₂ (at constant pressure and temperature).
The Ideal Gas Law: A Comprehensive Relationship
The Ideal Gas Law combines Boyle's Law, Charles's Law, and Avogadro's Law into a single equation that describes the relationship between pressure, volume, temperature, and the amount of gas:
PV = nRT
Where:
- P = pressure
- V = volume
- n = number of moles
- R = the ideal gas constant (a proportionality constant)
- T = temperature (in Kelvin)
This equation is incredibly useful for predicting the behavior of gases under various conditions. However, it's important to remember that the Ideal Gas Law is an approximation. Real gases deviate from ideal behavior, particularly at high pressures and low temperatures, where intermolecular forces become significant.
Real Gases vs. Ideal Gases: Deviations from Ideal Behavior
The Ideal Gas Law assumes that gas particles have negligible volume and no intermolecular forces. While this is a useful simplification for many situations, real gases do exhibit deviations from ideal behavior.
Factors Causing Deviations:
-
Intermolecular forces: Attractive forces between gas particles cause them to be slightly closer together than predicted by the Ideal Gas Law, leading to a smaller volume. Repulsive forces can have the opposite effect.
-
Finite volume of gas particles: The assumption of negligible particle volume breaks down at high pressures, where the actual volume of the particles becomes a significant fraction of the total volume.
The van der Waals Equation: A More Accurate Model
To account for these deviations, the van der Waals equation is often used. This equation includes correction terms to account for intermolecular forces (represented by 'a') and the finite volume of gas particles (represented by 'b'):
(P + a(n/V)²)(V - nb) = nRT
The van der Waals equation provides a more accurate description of real gas behavior, particularly at high pressures and low temperatures, where deviations from ideality are more pronounced.
Applications of Understanding Gas Volume
The understanding of gas volume and its relationship to pressure, temperature, and amount of gas has numerous applications in various fields:
1. Meteorology:
Weather forecasting relies heavily on understanding the behavior of gases in the atmosphere. Changes in pressure, temperature, and humidity directly affect the volume of atmospheric gases, influencing weather patterns.
2. Engineering:
Engineers use the principles of gas behavior in designing various systems, including internal combustion engines, gas pipelines, and refrigeration systems. Accurate predictions of gas volume are crucial for optimal design and operation.
3. Chemistry and Chemical Engineering:
Gas volume calculations are essential in stoichiometry, determining the amounts of reactants and products in chemical reactions involving gases. Chemical engineers utilize this knowledge in designing and optimizing industrial chemical processes.
4. Medicine:
Understanding gas behavior is important in various medical applications, such as respiratory therapy and anesthesia. The volume of gases in the lungs and their exchange with the blood are vital for proper respiratory function.
Conclusion: Gases and Their Adaptable Volumes
In conclusion, gases do not possess a fixed volume. Their volume is highly dependent on pressure, temperature, and the amount of gas present. The Ideal Gas Law provides a good approximation of gas behavior under many conditions, while the van der Waals equation offers a more accurate model for real gases exhibiting deviations from ideality. Understanding these principles is fundamental across many scientific and engineering disciplines. The compressibility and adaptability of gases are key characteristics that shape their behavior and applications in the world around us. The seemingly simple question of whether gases have a fixed volume opens a door to a fascinating exploration of the fundamental laws governing the physical world.
Latest Posts
Latest Posts
-
11 N 2 12 2n 1 Is Divisible By 133
Mar 15, 2025
-
The Figure Shows Two Charged Particles On An X Axis
Mar 15, 2025
-
Analysis Of Ode To The West Wind
Mar 15, 2025
-
What Is The Meaning Of The Term Threshold Stimulus
Mar 15, 2025
-
Which Of The Following Is True About A Firewall
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Do Gases Have A Fixed Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
