Do Gases Have A Definite Volume And Shape
News Leon
Mar 31, 2025 · 6 min read
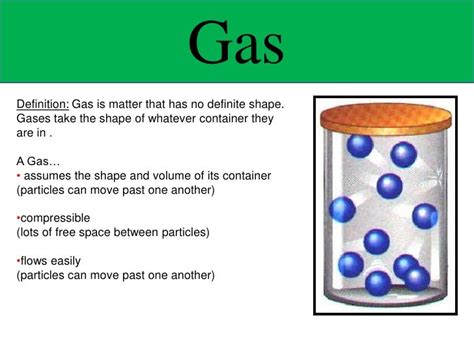
Table of Contents
Do Gases Have a Definite Volume and Shape? Exploring the Properties of Gases
Gases are one of the four fundamental states of matter, alongside solids, liquids, and plasmas. Understanding their unique properties, particularly their volume and shape, is crucial to comprehending various scientific concepts and real-world phenomena. Unlike solids and liquids, gases don't possess a definite volume or shape. This characteristic stems from the unique behavior of gas particles at the molecular level. This article delves deep into the properties of gases, explaining why they lack definite volume and shape, and exploring the factors that influence their behavior.
The Kinetic Molecular Theory of Gases: Understanding Gas Behavior
The behavior of gases is best explained using the Kinetic Molecular Theory (KMT). This theory proposes that gases consist of tiny particles (atoms or molecules) that are in constant, random motion. Several key postulates underpin this theory:
-
Gases are composed of tiny particles: These particles are significantly smaller than the distances between them. This means that the volume occupied by the particles themselves is negligible compared to the total volume of the gas.
-
Gas particles are in constant, random motion: They move in straight lines until they collide with each other or with the container walls. These collisions are perfectly elastic, meaning no kinetic energy is lost during the collisions.
-
The forces of attraction and repulsion between gas particles are negligible: This implies that the particles are essentially independent of each other and don't interact significantly unless they collide. This is a simplification, and real gases deviate from this ideal behavior at high pressures and low temperatures.
-
The average kinetic energy of gas particles is directly proportional to the absolute temperature: This means that as the temperature increases, the particles move faster.
Why Gases Don't Have a Definite Volume
The absence of a definite volume in gases is a direct consequence of the KMT. Because gas particles are in constant, random motion and the forces between them are negligible, they readily expand to fill the available space. If you place a gas in a small container, the particles will collide with the walls more frequently. If you transfer the same amount of gas to a larger container, the particles will spread out to fill the increased volume. The gas will always assume the volume of its container. There's no inherent volume that the gas "occupies" independently.
Factors Affecting Gas Volume: Pressure and Temperature
While gases don't have a definite volume in the sense of an inherent property, their volume can be significantly affected by external factors:
-
Pressure: Pressure is defined as the force exerted per unit area. Increasing the pressure on a gas forces the particles closer together, reducing the volume. Conversely, decreasing the pressure allows the gas to expand, increasing its volume. This relationship is described by Boyle's Law, which states that at a constant temperature, the volume of a gas is inversely proportional to its pressure (V ∝ 1/P).
-
Temperature: Increasing the temperature increases the kinetic energy of the gas particles, causing them to move faster and collide more frequently and forcefully with the container walls. This leads to an expansion in volume. Charles's Law describes this relationship: at a constant pressure, the volume of a gas is directly proportional to its absolute temperature (V ∝ T).
Why Gases Don't Have a Definite Shape
Similar to their volume, gases lack a definite shape due to the constant, random motion of their particles. Gas particles are not held together by strong intermolecular forces, unlike the particles in solids and liquids. Consequently, they readily adapt to the shape of their container. If you transfer a gas from a spherical container to a cubic container, the gas will instantly conform to the shape of the new container. The gas simply conforms to the boundaries of its environment.
Influence of Container Shape on Gas Distribution
The distribution of gas particles within a container is uniform, irrespective of the container's shape. This uniformity arises from the continuous random motion of the particles. Regardless of whether the container is spherical, cubic, or any other shape, the gas particles will distribute themselves evenly throughout the available space, maximizing entropy (randomness).
Ideal Gases vs. Real Gases: Deviations from Ideal Behavior
The Kinetic Molecular Theory describes the behavior of ideal gases. Ideal gases are a theoretical concept where the postulates of the KMT are perfectly followed. However, real gases deviate from ideal behavior, particularly under conditions of high pressure and low temperature.
At high pressures, the gas particles are forced close together, and the volume occupied by the particles themselves becomes significant relative to the total volume of the gas. This leads to a decrease in the volume of the gas compared to what would be predicted by the ideal gas law.
At low temperatures, the intermolecular attractive forces between the gas particles become more significant. These forces cause the particles to cluster together, reducing their random motion and affecting the volume and pressure.
The van der Waals equation is an improved model that accounts for these deviations from ideal behavior by incorporating correction factors for intermolecular forces and the volume occupied by the gas particles.
Applications and Real-World Examples
The understanding of gas behavior is crucial in numerous scientific and engineering applications:
-
Weather forecasting: Meteorologists utilize the principles of gas behavior to predict weather patterns, as the atmosphere is predominantly composed of gases. Changes in temperature, pressure, and humidity directly influence atmospheric volume and gas distribution, leading to various weather phenomena.
-
Aerospace engineering: The design and operation of aircraft and rockets rely heavily on understanding gas dynamics, especially concerning the behavior of gases in combustion engines and jet propulsion systems.
-
Chemical engineering: Numerous industrial processes involve gases, including the production of chemicals, refining of petroleum, and manufacturing of various materials. Understanding gas behavior is crucial for optimizing these processes and ensuring safety.
-
Medical applications: Understanding gas behavior is vital in various medical fields, such as respiratory therapy, anesthesia, and diving medicine. It helps explain lung function and the effects of gases on the human body at different pressures and depths.
-
Environmental science: The understanding of atmospheric gases and their behavior is critical for environmental studies, including climate change research, air pollution monitoring, and understanding the greenhouse effect.
Conclusion: Understanding the Dynamic Nature of Gases
In conclusion, gases do not have a definite volume or shape because of the unique behavior of their constituent particles as described by the Kinetic Molecular Theory. These particles are in constant, random motion, and the forces between them are generally negligible, allowing them to expand to fill the available space and readily adopt the shape of their container. While ideal gas laws provide a good approximation of gas behavior under many conditions, it's important to remember that real gases deviate from ideal behavior, particularly at high pressures and low temperatures. Understanding these properties is crucial across diverse scientific disciplines and practical applications, underscoring the importance of comprehending the dynamic nature of gases.
Latest Posts
Latest Posts
-
Why Nh3 Is More Polar Than Nf3
Apr 02, 2025
-
Abraham Maslows Needs Theory Of Motivation Assumes That
Apr 02, 2025
-
Pb Oh 2 Hcl H2o Pbcl2
Apr 02, 2025
-
Is Ethanol More Polar Than Water
Apr 02, 2025
-
Cis 1 Tert Butyl 2 Methylcyclohexane
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Do Gases Have A Definite Volume And Shape . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
