Do Gases Have A Definite Volume
News Leon
Mar 27, 2025 · 6 min read
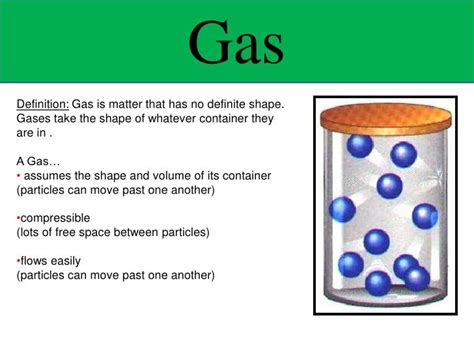
Table of Contents
Do Gases Have a Definite Volume? Exploring the Nature of Gases
The question of whether gases possess a definite volume is a fundamental concept in chemistry and physics. The short answer is no, gases do not have a definite volume. Unlike solids and liquids, which maintain a relatively fixed shape and volume, gases are highly compressible and readily expand to fill the container they occupy. Understanding this characteristic requires delving into the kinetic molecular theory of gases and exploring the factors that influence gas behavior.
Understanding the Kinetic Molecular Theory of Gases
The kinetic molecular theory (KMT) provides a microscopic explanation for the macroscopic properties of gases. This theory rests on several key postulates:
-
Gases are composed of tiny particles (atoms or molecules) that are in constant, random motion. These particles are in a state of perpetual movement, colliding with each other and the walls of their container. This constant motion is the driving force behind many of the properties of gases.
-
The volume of the gas particles themselves is negligible compared to the total volume of the gas. This means that the space occupied by the gas particles is insignificant relative to the vast empty space between them. This is especially true at low pressures and high temperatures.
-
There are no significant attractive or repulsive forces between gas particles. The particles are considered to be independent of each other, interacting only through collisions. This assumption is most accurate for ideal gases, although real gases exhibit some intermolecular forces.
-
Collisions between gas particles and between particles and the container walls are elastic. This means that no kinetic energy is lost during collisions. The total kinetic energy of the system remains constant.
-
The average kinetic energy of the gas particles is directly proportional to the absolute temperature (in Kelvin). As temperature increases, the average speed of the particles increases, leading to more frequent and forceful collisions.
Why Gases Don't Have a Definite Volume: The Role of Compressibility
The lack of a definite volume in gases is a direct consequence of the KMT postulates. Because the gas particles are widely dispersed and the intermolecular forces are weak, the gas readily expands to fill the available space. Applying pressure to a gas reduces the volume, forcing the particles closer together. This is why gases are highly compressible. Conversely, reducing the pressure allows the gas to expand, increasing its volume.
Think of it like this: Imagine a balloon filled with air. The air molecules are free to move within the confines of the balloon. If you increase the size of the balloon, the air molecules will spread out to fill the larger space. The volume of the air has changed, but the amount of air (the number of molecules) remains constant. If you squeeze the balloon, you decrease the volume, compressing the air molecules closer together.
This contrasts sharply with solids and liquids. In solids, the particles are tightly packed together in a fixed arrangement, resulting in a definite volume. In liquids, the particles are closer together than in gases but still relatively free to move past one another. While liquids can be slightly compressed, they are far less compressible than gases.
Factors Affecting Gas Volume: Pressure, Temperature, and Amount
Several factors influence the volume of a gas:
Pressure:
Pressure is the force exerted per unit area by gas particles colliding with the walls of their container. Boyle's Law describes the inverse relationship between pressure and volume at a constant temperature: as pressure increases, volume decreases, and vice versa. This is because increased pressure forces the gas particles closer together, reducing the volume.
Temperature:
Temperature affects the kinetic energy of the gas particles. Charles's Law states that at constant pressure, the volume of a gas is directly proportional to its absolute temperature. As temperature increases, the kinetic energy of the gas particles increases, causing them to move faster and collide more frequently and forcefully. This leads to an increase in volume.
Amount of Gas:
The number of gas particles also affects the volume. Avogadro's Law states that at constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of gas. More gas particles mean more collisions and a larger volume.
The Ideal Gas Law: Combining the Factors
The Ideal Gas Law, PV = nRT, combines the relationships between pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). This equation is a powerful tool for predicting the behavior of gases under various conditions. It's important to remember that the ideal gas law is a simplification and doesn't perfectly describe the behavior of all gases under all conditions, especially at high pressures or low temperatures where intermolecular forces become significant.
Real Gases vs. Ideal Gases: Deviations from the Ideal Gas Law
The Ideal Gas Law assumes that gas particles have negligible volume and exert no intermolecular forces. However, real gases deviate from this ideal behavior, especially at high pressures and low temperatures.
At high pressures, the volume of the gas particles themselves becomes significant relative to the total volume of the gas. This reduces the available space for the particles to move, leading to a smaller volume than predicted by the Ideal Gas Law.
At low temperatures, intermolecular attractive forces become more significant. These forces pull the particles closer together, reducing the volume and increasing the density of the gas.
These deviations from ideal behavior are accounted for by using more complex equations of state, such as the van der Waals equation, which incorporates corrections for particle volume and intermolecular forces.
Applications and Implications of Understanding Gas Volume
Understanding the behavior of gases and their lack of definite volume has numerous practical applications across various scientific and engineering disciplines:
-
Weather forecasting: The atmospheric pressure, temperature, and humidity (which influences the amount of water vapor) are crucial in predicting weather patterns. Understanding how these factors affect the volume and movement of air masses is essential.
-
Aerospace engineering: Designing aircraft and spacecraft requires precise calculations of gas behavior under various conditions, including changes in altitude and temperature.
-
Chemical engineering: Many industrial processes involve gases, and controlling their volume and pressure is crucial for efficient and safe operation. This includes processes such as chemical synthesis, gas separation, and combustion.
-
Medical applications: Understanding the behavior of respiratory gases (oxygen and carbon dioxide) in the lungs and bloodstream is fundamental to respiratory medicine and the treatment of respiratory diseases. This also extends to the use of gases in medical imaging and treatment.
Conclusion
Gases do not have a definite volume. Their volume is highly dependent on pressure, temperature, and the amount of gas present. This characteristic stems from the kinetic molecular theory of gases, which describes gases as collections of particles in constant, random motion with negligible intermolecular forces. While the ideal gas law provides a useful simplification, real gases deviate from ideal behavior under certain conditions. Understanding the behavior of gases is essential in various fields, influencing our comprehension of atmospheric phenomena, the design of engineering systems, and advancements in medicine and other scientific areas. The ability to predict and control gas volume is vital to countless applications, emphasizing the importance of this fundamental concept in our understanding of the physical world.
Latest Posts
Latest Posts
-
How Many Electrons Does Antimony Have
Mar 30, 2025
-
Pericardial Fluid Is Found Between The And The
Mar 30, 2025
-
What Is The Angular Velocity Of The Earth
Mar 30, 2025
-
Which Layer Does Weather Occur In
Mar 30, 2025
-
Economic Growth Is Depicted By
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Do Gases Have A Definite Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
