Difference Between Ideal Gas And Non Ideal Gas
News Leon
Mar 18, 2025 · 6 min read
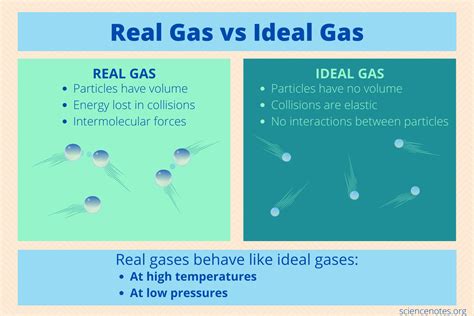
Table of Contents
The Great Divide: Understanding the Difference Between Ideal and Non-Ideal Gases
The world of gases is vast and complex, encompassing everything from the air we breathe to the propellants in aerosol cans. To understand their behavior, we often employ models. Two crucial models are the ideal gas and the non-ideal gas. While the ideal gas model simplifies calculations, the non-ideal gas model provides a more accurate representation of real-world gases. This article delves into the significant differences between these two models, exploring their underlying assumptions, equations, and applications.
Ideal Gas: A Simplified Model
The ideal gas model simplifies the complexities of molecular interactions. It rests on several key assumptions:
Assumptions of the Ideal Gas Law
- Negligible Molecular Volume: Ideal gas molecules are considered point masses with negligible volume compared to the volume of the container. This implies that the molecules themselves don't occupy any significant space.
- No Intermolecular Forces: There are no attractive or repulsive forces between gas molecules. They move independently, without interacting with each other. This means collisions are perfectly elastic, with no energy loss.
- Random Motion: Gas molecules move randomly in all directions with constant, straight-line motion between collisions.
- Elastic Collisions: Collisions between gas molecules and the container walls are perfectly elastic. Kinetic energy is conserved during collisions.
These assumptions, while simplifying, allow for the derivation of the ideal gas law, a fundamental equation in chemistry and physics:
PV = nRT
Where:
- P represents pressure
- V represents volume
- n represents the number of moles of gas
- R is the ideal gas constant (a constant value that depends on the units used for pressure, volume, and temperature)
- T represents temperature (in Kelvin)
The ideal gas law provides a simple yet powerful tool for predicting the behavior of gases under various conditions. However, it's crucial to remember that it's a simplification; real gases deviate from this idealized behavior.
Non-Ideal Gas: A More Realistic Representation
Real gases deviate from ideal behavior, especially at high pressures and low temperatures. This is because the assumptions of the ideal gas model break down under these conditions.
Deviations from Ideality
- Significant Molecular Volume: At high pressures, gas molecules are compressed into a smaller volume. Their own volume becomes a significant fraction of the total volume, and the assumption of negligible volume no longer holds.
- Intermolecular Forces: At low temperatures, the kinetic energy of the gas molecules is reduced. This allows intermolecular forces (like van der Waals forces) to become significant, affecting the motion and collisions of molecules. Attractive forces cause deviations from ideal behavior, while repulsive forces can also lead to deviations at extremely high pressures.
- Non-Elastic Collisions: While the ideal gas law assumes perfectly elastic collisions, real collisions are rarely perfectly elastic. Some energy may be lost or gained during a collision, altering the overall kinetic energy of the system.
The Compressibility Factor (Z)
To quantify the deviation of a real gas from ideal behavior, we use the compressibility factor (Z). Z is defined as the ratio of the molar volume of a real gas to the molar volume of an ideal gas under the same conditions of temperature and pressure:
Z = V<sub>real</sub> / V<sub>ideal</sub>
- For an ideal gas, Z = 1.
- For real gases, Z can be greater than, less than, or equal to 1, depending on the temperature and pressure. Values of Z significantly different from 1 indicate substantial deviations from ideal behavior.
Equations of State for Non-Ideal Gases
Several equations of state have been developed to better model non-ideal gases. These equations incorporate correction factors to account for the limitations of the ideal gas law. The most well-known is the van der Waals equation:
(P + a(n/V)²)(V - nb) = nRT
Where:
- a and b are van der Waals constants specific to each gas. 'a' accounts for intermolecular attractive forces, and 'b' accounts for the volume occupied by the gas molecules.
The van der Waals equation provides a more accurate representation of real gases, particularly at moderate pressures and temperatures. However, even this improved equation has limitations and may not accurately predict behavior under all conditions. Other equations of state, like the Redlich-Kwong and Peng-Robinson equations, offer even greater accuracy for specific types of gases and conditions but are more complex.
Comparing Ideal and Non-Ideal Gases: A Table Summary
| Feature | Ideal Gas | Non-Ideal Gas |
|---|---|---|
| Molecular Volume | Negligible | Significant, especially at high pressure |
| Intermolecular Forces | None | Present (attractive and/or repulsive) |
| Collisions | Perfectly elastic | Not perfectly elastic |
| Equation of State | PV = nRT | van der Waals, Redlich-Kwong, etc. |
| Compressibility Factor (Z) | 1 | Deviates from 1 |
| Conditions of Applicability | Low pressure, high temperature | High pressure, low temperature |
| Accuracy | Approximative | More accurate, but more complex |
Applications and Significance
Understanding the difference between ideal and non-ideal gases has significant implications in various fields:
Chemical Engineering
Accurate modeling of gas behavior is essential in chemical processes involving gas mixtures, such as refining, petrochemical production, and gas liquefaction. Non-ideal gas equations are critical for accurate design and optimization of these processes.
Environmental Science
Atmospheric modeling requires considering the non-ideal behavior of gases present in the atmosphere, particularly at high altitudes and under varying temperature and pressure conditions. This is crucial for understanding climate change and air pollution.
Physics
In physics, particularly in areas like thermodynamics and statistical mechanics, the ideal gas model serves as a foundational concept. However, understanding deviations from ideality is important for accurate descriptions of real-world phenomena.
Medical Applications
Understanding gas behavior plays a role in medical applications, such as respiratory therapy and anesthesia. The solubility and diffusion of gases in the body depend on their properties, which deviate from ideal gas behavior under physiological conditions.
Conclusion: Choosing the Right Model
The ideal gas law offers a simple and useful approximation for gas behavior under many conditions. However, for situations where pressure is high, temperature is low, or high accuracy is crucial, the non-ideal gas models, and their respective equations of state, must be employed. The choice between the ideal and non-ideal models depends on the specific application and the desired level of accuracy. Understanding the limitations and assumptions of each model is vital for accurate predictions and meaningful interpretations of gas behavior in various scientific and engineering applications. Remember, the ideal gas is a tool, and like any tool, its effectiveness depends on its proper application.
Latest Posts
Latest Posts
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Ideal Gas And Non Ideal Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
