Describe The Electron-sea Model Of Metallic Bonding
News Leon
Mar 14, 2025 · 6 min read
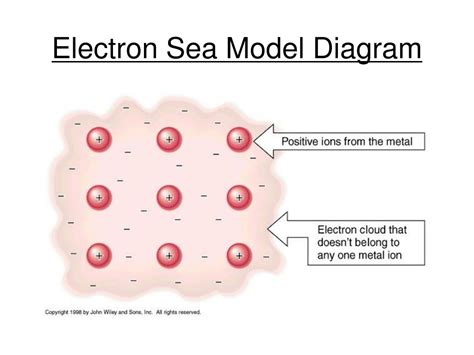
Table of Contents
Delving Deep into the Electron Sea Model: Understanding Metallic Bonding
The shimmering gleam of gold, the robust strength of steel, the excellent conductivity of copper – these properties, and countless others, are all a direct result of metallic bonding. Unlike the directional covalent bonds or the electrostatic attraction in ionic compounds, metals exhibit a unique type of bonding best described by the electron sea model. This model, a cornerstone of materials science, provides a simplified yet insightful explanation of the remarkable characteristics of metallic materials. This article will thoroughly explore the electron sea model, examining its principles, strengths, limitations, and its implications in understanding the macroscopic properties of metals.
The Fundamentals of the Electron Sea Model
At its core, the electron sea model depicts a metal as a lattice of positively charged metal ions submerged in a "sea" of delocalized valence electrons. Instead of being tightly bound to individual atoms as in covalent bonding, these valence electrons are free to move throughout the entire metal structure. This mobility is the key to understanding the unique properties of metals. Think of it like a "jelly" where the positive metal ions are the "fruit" embedded within a mobile, negatively charged "jelly" of electrons.
Delocalized Electrons: The Heart of the Model
The crucial concept here is delocalization. In contrast to localized electrons in covalent bonds, the valence electrons in metals are not associated with any particular atom. They're free to roam across the entire crystal lattice, creating a collective electron cloud that binds the positive ions together. This "sea" of electrons acts as a kind of "glue," holding the metal structure together through electrostatic attraction.
The Role of Valence Electrons
The number of valence electrons contributed by each metal atom significantly influences the properties of the metal. Metals with more valence electrons generally exhibit stronger metallic bonding and higher melting points. This is because a larger number of delocalized electrons results in a stronger electrostatic attraction between the electron sea and the positive metal ions. For instance, transition metals, with multiple valence electrons, often possess exceptional strength and high melting points compared to alkali metals with only one valence electron.
Explaining Metallic Properties with the Electron Sea Model
The electron sea model elegantly explains many of the characteristic properties of metals:
1. Electrical Conductivity:
The most striking feature of metals is their excellent electrical conductivity. The freely moving electrons in the electron sea readily respond to an applied electric field. When a voltage is applied across a metal, these delocalized electrons flow through the material, carrying the electric current. This explains why metals are such efficient conductors of electricity. The ease of electron movement is directly related to the conductivity; the more mobile the electrons, the higher the conductivity.
2. Thermal Conductivity:
Metals are also excellent conductors of heat. This is again a direct consequence of the delocalized electrons. When one part of a metal is heated, the increased kinetic energy of the electrons is rapidly transferred throughout the material by the movement of these electrons, leading to efficient heat conduction. The thermal conductivity is directly correlated with the electrical conductivity, a phenomenon known as the Wiedemann-Franz law.
3. Malleability and Ductility:
Metals are known for their malleability (ability to be hammered into sheets) and ductility (ability to be drawn into wires). The electron sea model explains this. When a metal is deformed, the layers of metal ions can slide past each other without breaking the metallic bonds. This is because the delocalized electrons readily adjust their positions, maintaining the electrostatic attraction between the ions even as the lattice structure changes. This "electron glue" allows for significant deformation without causing the material to fracture.
4. Metallic Luster:
The characteristic shiny appearance of metals, known as metallic luster, arises from the interaction of light with the delocalized electrons. The electrons absorb and re-emit light across a wide range of frequencies, giving metals their characteristic luster. The free electrons effectively scatter incident light, resulting in the reflection of visible light and the appearance of shine.
5. High Melting and Boiling Points (Generally):
While not universally true across all metals, many metals exhibit relatively high melting and boiling points. This is attributed to the strong electrostatic attraction between the sea of electrons and the positively charged metal ions. Overcoming this attraction requires significant energy, leading to higher melting and boiling points compared to substances with weaker intermolecular forces. However, it's crucial to note that the strength of the metallic bond varies significantly depending on the specific metal and its electronic structure.
Limitations of the Electron Sea Model
While remarkably successful in explaining many metallic properties, the electron sea model has its limitations:
1. Simplification of Electron Behavior:
The model simplifies the complex quantum mechanical behavior of electrons. It treats electrons as a uniform sea, neglecting the variations in electron energy levels and their wave-like nature. A more accurate description requires the use of band theory, which considers the quantum mechanical behavior of electrons in a periodic potential.
2. Doesn't Explain All Magnetic Properties:
The electron sea model struggles to fully explain the magnetic properties of metals, especially ferromagnetism. This requires a more sophisticated understanding of electron spin and interactions between electron spins within the metal lattice.
3. Doesn't Account for Alloying Behavior:
While the model can provide a general understanding of alloys, it doesn't fully explain the complexities of alloy formation and the resulting changes in properties. More detailed models are needed to account for the interactions between different metal atoms in an alloy.
4. Fails to Predict Precise Properties:
While the model qualitatively explains various properties, it doesn't provide quantitative predictions for specific physical properties. For precise calculations, more complex computational methods and band theory are necessary.
Beyond the Electron Sea: Band Theory
A more sophisticated model that overcomes some of the limitations of the electron sea model is band theory. Band theory builds upon the principles of quantum mechanics and provides a more detailed description of electron behavior in metals. It considers the interaction of atomic orbitals to form energy bands. These energy bands describe the allowed energy levels for electrons in the solid state. The delocalized electrons occupy these energy bands, with the highest occupied energy band known as the valence band. The presence of a partially filled valence band or an overlapping valence and conduction band is what allows for the high electrical conductivity in metals.
Band theory provides a more precise and quantitative description of metallic properties compared to the electron sea model, especially concerning electrical conductivity, optical properties, and the prediction of various material parameters.
Conclusion: A Valuable Simplified Model
Despite its limitations, the electron sea model remains an invaluable tool for understanding the fundamental nature of metallic bonding. Its simplicity makes it an excellent starting point for learning about the unique properties of metals and offers an intuitive understanding of their behavior. While more advanced models like band theory provide more detailed and accurate descriptions, the electron sea model continues to serve as a powerful conceptual framework for grasping the essence of metallic bonding and the remarkable properties that define this crucial class of materials. Its ability to explain electrical conductivity, thermal conductivity, malleability, ductility, and metallic luster makes it a cornerstone in materials science education and a crucial stepping stone to more advanced concepts in solid-state physics. Understanding the electron sea model provides a strong foundation for further exploration of the fascinating world of materials science and engineering.
Latest Posts
Latest Posts
-
What Capacitance Is Required To Store An Energy Of
Mar 14, 2025
-
What Is The Major Product Of The Following Reaction Sequence
Mar 14, 2025
-
How Many Lightyears Is The Sun
Mar 14, 2025
-
Convert Timestamp To Date Time Python
Mar 14, 2025
-
Difference Between A Monologue And A Soliloquy
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Describe The Electron-sea Model Of Metallic Bonding . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
