Coordination Number Of Hexagonal Close Packing
News Leon
Apr 01, 2025 · 5 min read
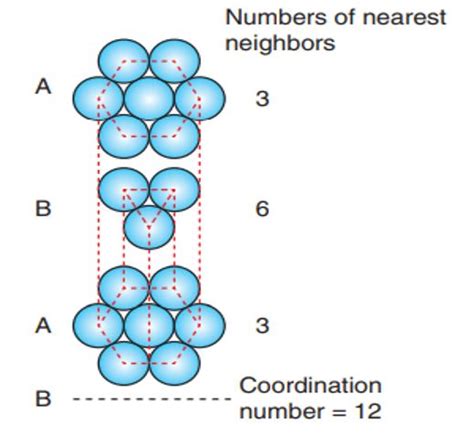
Table of Contents
Coordination Number of Hexagonal Close Packing (HCP)
Hexagonal close packing (HCP) is a common crystal structure found in many metals and alloys. Understanding its coordination number is crucial for predicting material properties like density, ductility, and thermal conductivity. This article delves deep into the intricacies of HCP, explaining its structure and meticulously calculating its coordination number. We will explore different approaches to understanding this key characteristic, clarifying any potential misconceptions along the way.
Understanding Hexagonal Close Packing (HCP)
Before diving into the coordination number, let's establish a solid understanding of the HCP structure itself. Imagine arranging spheres in a layer, each sphere nestled in the depression formed by three adjacent spheres. This forms a triangular lattice.
The ABAB Stacking Sequence
Now, let's add another layer on top. There are two possible positions for the spheres in the second layer. They can either sit directly above the spheres in the first layer (AA stacking), or they can sit in the depressions between the spheres in the first layer (AB stacking). In HCP, the stacking sequence is ABABAB… This means the third layer sits directly above the first, the fourth above the second, and so on. This creates a structure with a hexagonal unit cell.
Visualizing the Structure
Visualizing HCP can be challenging. Imagine stacking coins. The first layer forms a hexagonal arrangement. The second layer fits into the depressions of the first. The third layer mirrors the first, and so on. This ABAB stacking sequence is what distinguishes HCP from cubic close packing (CCP), which has an ABCABC… stacking sequence.
Calculating the Coordination Number
The coordination number refers to the number of nearest neighbors surrounding a given atom in a crystal structure. Determining this number for HCP requires a systematic approach.
Method 1: Direct Counting from the Unit Cell
Consider a single atom within the HCP unit cell. Let's carefully count its nearest neighbors.
- Layer 1: The central atom is surrounded by six atoms in its own layer.
- Layer 2: Three atoms from the layer above and three from the layer below are its nearest neighbors.
- Total: 6 (layer 1) + 3 (layer above) + 3 (layer below) = 12
Therefore, using this direct counting method within the unit cell, the coordination number of HCP is 12.
Method 2: Considering the Packing Efficiency
Another way to understand the coordination number is to consider the packing efficiency of HCP. HCP, like CCP, achieves the highest possible packing efficiency for spheres of equal size, which is approximately 74%. This high packing efficiency is a direct consequence of each atom being surrounded by 12 nearest neighbors. This high density also implies a high coordination number. The high coordination number is what contributes to the structure's strength and stability.
Method 3: Analyzing the Atomic Radii and Distances
Analyzing the distances between the central atom and its surrounding atoms further validates the coordination number. All 12 nearest neighbors are equidistant from the central atom. This uniform distance to the nearest neighbors is a key characteristic of close-packed structures like HCP and CCP, both of which have a coordination number of 12. Any atom further away would not be considered a nearest neighbor.
Comparison with Other Crystal Structures
Let's compare the coordination number of HCP with other common crystal structures:
| Crystal Structure | Coordination Number | Packing Efficiency |
|---|---|---|
| Simple Cubic (SC) | 6 | 52% |
| Body-Centered Cubic (BCC) | 8 | 68% |
| Face-Centered Cubic (FCC) / Cubic Close Packing (CCP) | 12 | 74% |
| Hexagonal Close Packing (HCP) | 12 | 74% |
This table highlights that HCP and FCC both have the highest coordination numbers and packing efficiencies among common crystal structures. This high coordination directly influences their physical and mechanical properties.
Significance of the Coordination Number
The coordination number of 12 in HCP has profound implications on the material's properties:
Density
The high coordination number leads to a high atomic packing density, resulting in a relatively high density for HCP materials. This density influences applications where high mass-to-volume ratios are desirable.
Mechanical Properties
The strong bonding associated with the 12 nearest neighbors contributes to the relatively high strength and ductility of many HCP metals. However, the anisotropy of HCP (its properties vary depending on the crystallographic direction) can also lead to less ductility along certain directions.
Thermal and Electrical Conductivity
The close proximity of atoms in HCP structures facilitates efficient transfer of heat and electrical energy, contributing to comparatively high thermal and electrical conductivities in some HCP metals.
Implications for Material Selection
Understanding the coordination number is crucial in material selection for engineering applications. The high coordination number and packing efficiency in HCP make materials with this structure suitable for applications requiring high strength, density, or thermal conductivity. However, the anisotropy inherent in the structure needs to be considered.
Potential Misconceptions and Clarifications
A common misconception is that the coordination number is simply the number of atoms touching a given atom. While this is often true, it's important to consider the distance. In HCP, the 12 nearest neighbors are all equidistant, confirming the coordination number of 12. Atoms further away, even if they are touching, are not considered nearest neighbors.
Advanced Concepts and Further Exploration
The coordination number is a fundamental concept in crystallography, but further exploration can delve into:
- Interstitial sites: These are spaces between the atoms in the HCP lattice where smaller atoms can reside. Understanding these sites is crucial in the study of alloys and interstitial compounds.
- Defects in HCP structures: Like all crystals, HCP structures can contain defects that influence their properties. Studying these defects helps us understand real-world material behavior.
- Simulations and modeling: Computational methods are used to simulate the atomic-level behavior of HCP materials and to predict their properties.
Conclusion
The coordination number of hexagonal close packing is definitively 12. This fundamental characteristic stems from its unique ABAB stacking sequence and leads to important material properties such as high density, strength, and conductivity. Understanding the coordination number and its implications is vital for anyone working with materials science, crystallography, or related fields. It's a cornerstone concept in predicting and explaining the behavior of a wide range of materials. Further investigation into related concepts will provide a deeper and more nuanced understanding of the complexity and beauty of crystal structures.
Latest Posts
Latest Posts
-
What Is 13 25 As A Decimal
Apr 02, 2025
-
How Many Months Are In Five Years
Apr 02, 2025
-
Geometric Mean Of 9 And 4
Apr 02, 2025
-
A Codon Consists Of How Many Bases
Apr 02, 2025
-
All Summer In A Day Ray Bradbury Summary
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Coordination Number Of Hexagonal Close Packing . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
