Chemical Formula Of Nitrogen And Hydrogen
News Leon
Mar 17, 2025 · 6 min read
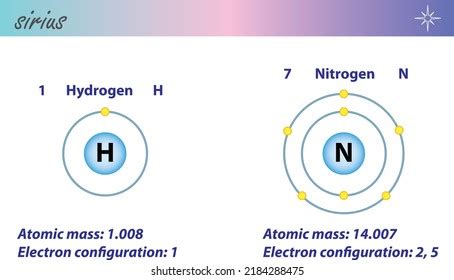
Table of Contents
The Chemical Formulas of Nitrogen and Hydrogen: A Deep Dive
Nitrogen and hydrogen, two of the most abundant elements in the universe, play crucial roles in various natural processes and industrial applications. Understanding their chemical formulas and properties is fundamental to comprehending their diverse functionalities. This comprehensive article delves into the chemical formulas of nitrogen and hydrogen, exploring their individual characteristics, reactions, and significance in different contexts.
Understanding the Chemical Formula of Nitrogen (N₂)
Nitrogen, represented by the symbol 'N' and atomic number 7, exists primarily as a diatomic molecule, meaning two nitrogen atoms are bonded together. This is the key to understanding its chemical formula: N₂. This strong triple bond between the two nitrogen atoms is responsible for its relative inertness at standard temperature and pressure.
Properties of Nitrogen Gas (N₂)
-
Gas at Room Temperature: Nitrogen exists as a colorless, odorless, and tasteless gas at room temperature and pressure. Its inertness is a direct consequence of the strong triple bond in the N₂ molecule, making it difficult to break.
-
Low Reactivity: This inherent stability makes nitrogen a relatively unreactive element under normal conditions. It's this low reactivity that makes it crucial for various applications where a non-reactive atmosphere is required.
-
Triple Bond Strength: The triple bond (N≡N) between the two nitrogen atoms is exceptionally strong, requiring significant energy to break. This high bond dissociation energy contributes to its inertness.
-
Abundance in the Atmosphere: Nitrogen constitutes approximately 78% of Earth's atmosphere, making it the most abundant element in the air we breathe. This abundance makes it a readily available resource for industrial applications.
-
Essential for Life: Despite its inertness, nitrogen is crucial for life. It's a vital component of amino acids, proteins, and nucleic acids – the building blocks of life. However, atmospheric nitrogen needs to be fixed (converted into usable forms) by biological processes or industrial methods before it can be utilized by organisms.
Industrial Applications of Nitrogen
The inert nature of nitrogen gas makes it invaluable in various industrial processes:
-
Food Preservation: Nitrogen is used to create a modified atmosphere packaging (MAP) for foods, extending shelf life by preventing oxidation and microbial growth.
-
Chemical Manufacturing: It serves as an inert atmosphere in chemical reactions, protecting reactive materials from oxidation or unwanted side reactions.
-
Electronics Industry: Used to purge oxygen and moisture from semiconductor manufacturing environments, ensuring high-quality electronic components.
-
Metal Production: Nitrogen is employed in the production of stainless steel and other specialized alloys, preventing oxidation and improving the material properties.
Understanding the Chemical Formula of Hydrogen (H₂)
Hydrogen, denoted by the symbol 'H' and atomic number 1, is the lightest element in the periodic table. Like nitrogen, it typically exists as a diatomic molecule, its chemical formula being H₂. Unlike nitrogen’s triple bond, hydrogen atoms are connected by a single covalent bond.
Properties of Hydrogen Gas (H₂)
-
Colorless, Odorless, Tasteless Gas: Hydrogen is a colorless, odorless, and tasteless gas at room temperature and pressure. Its simple molecular structure contributes to its properties.
-
Highly Flammable: Hydrogen is extremely flammable and reacts explosively with oxygen, forming water (H₂O). This high flammability necessitates careful handling and storage precautions.
-
Low Density: Hydrogen has the lowest density of all gases, making it an attractive fuel source for lighter-than-air vehicles (though its flammability poses significant challenges).
-
Abundance in the Universe: Hydrogen is the most abundant element in the universe, constituting the majority of the mass of stars. On Earth, it is found primarily in compounds such as water and hydrocarbons.
-
Versatile Reactant: Hydrogen readily reacts with many elements, forming various compounds, including water, ammonia, and numerous organic molecules. This reactivity makes it a cornerstone in many industrial chemical processes.
Industrial Applications of Hydrogen
The diverse reactivity of hydrogen makes it essential in numerous industrial processes:
-
Ammonia Production (Haber-Bosch Process): The most significant industrial application of hydrogen is in the production of ammonia (NH₃), primarily used in fertilizers.
-
Petroleum Refining: Hydrogen is used in petroleum refining processes to upgrade heavy crude oil fractions, converting them into lighter, more valuable products.
-
Metal Refining: Used in the refining of metals such as copper and nickel, removing impurities and improving the purity of the final product.
-
Fuel Cells: Hydrogen is employed in fuel cells to generate electricity through a chemical reaction with oxygen, producing only water as a byproduct—a clean energy source.
-
Food Processing: Hydrogenation is a process that uses hydrogen to convert liquid vegetable oils into solid fats, common in margarine production.
Reactions Involving Nitrogen and Hydrogen: The Haber-Bosch Process
The Haber-Bosch process is a prime example of a reaction involving both nitrogen and hydrogen. This industrial process is crucial for the production of ammonia, a fundamental component of fertilizers and other industrial chemicals.
The Haber-Bosch Reaction
The reaction can be represented by the following chemical equation:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
This equation shows that one molecule of nitrogen gas reacts with three molecules of hydrogen gas to produce two molecules of ammonia gas. The reaction is reversible and exothermic, meaning it releases heat.
Optimizing the Haber-Bosch Process
To maximize ammonia production, several factors need to be optimized:
-
High Pressure: High pressure shifts the equilibrium towards the product (ammonia) due to Le Chatelier's principle.
-
Moderate Temperature: A moderate temperature is used. While lower temperatures favor the equilibrium, the reaction rate becomes too slow. Higher temperatures increase the rate but reduce the yield.
-
Catalyst: An iron catalyst is used to speed up the reaction without affecting the equilibrium.
Comparing and Contrasting Nitrogen and Hydrogen
While both nitrogen and hydrogen are diatomic gases, their properties and applications differ significantly:
| Feature | Nitrogen (N₂) | Hydrogen (H₂) |
|---|---|---|
| Abundance | Most abundant in Earth's atmosphere | Most abundant in the universe |
| Reactivity | Relatively inert | Highly reactive |
| Bond Type | Triple covalent bond | Single covalent bond |
| Flammability | Non-flammable | Highly flammable |
| Industrial Use | Inert atmosphere, food preservation | Ammonia production, fuel cells |
Environmental Considerations
While both nitrogen and hydrogen are vital for many applications, their production and use have environmental implications:
-
Nitrogen Oxides (NOx): Combustion processes involving nitrogen can produce NOx, which contribute to air pollution and acid rain.
-
Ammonia Runoff: Excessive use of nitrogen-based fertilizers can lead to ammonia runoff into waterways, causing eutrophication and harming aquatic life.
-
Hydrogen Production: The production of hydrogen can be energy-intensive and can release greenhouse gases depending on the method used. Exploring sustainable hydrogen production methods is crucial for minimizing its environmental impact.
Future Prospects
Research and development continue to explore new applications and production methods for both nitrogen and hydrogen:
-
Green Ammonia Production: The pursuit of green ammonia production methods, utilizing renewable energy sources and minimizing greenhouse gas emissions, is gaining momentum.
-
Hydrogen as a Fuel: Hydrogen fuel cells are being increasingly researched and developed as a clean energy alternative, aiming to address the challenges associated with its storage and transportation.
-
Nitrogen Fixation Technologies: Developing more efficient and sustainable nitrogen fixation methods is critical to meet the increasing demand for nitrogen-based fertilizers while minimizing environmental impact.
In conclusion, understanding the chemical formulas of nitrogen (N₂) and hydrogen (H₂) is crucial for appreciating their individual properties and their combined roles in various industrial processes and natural phenomena. Further research and technological advancements are continuously expanding the applications of these essential elements, while also striving to mitigate their environmental impacts. The future of nitrogen and hydrogen hinges on developing sustainable production methods and exploring new applications that contribute to a cleaner and more sustainable world.
Latest Posts
Latest Posts
-
Predict What Is Present In Each Of The Following
Mar 18, 2025
-
Which Of The Following Word Is Different From The Others
Mar 18, 2025
-
Reverse List Python Without Inbuilt Function
Mar 18, 2025
-
A Current Of One Ampere Is Passed Through
Mar 18, 2025
-
Difference Between Earthing And Grounding And Neutral
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Chemical Formula Of Nitrogen And Hydrogen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
