Cells Shrink When They Are Placed In Solutions That Are
News Leon
Mar 23, 2025 · 6 min read
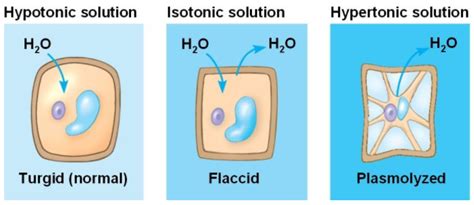
Table of Contents
Cells Shrink When Placed in Hypertonic Solutions: A Deep Dive into Osmosis
Cells are the fundamental units of life, tiny powerhouses carrying out a multitude of functions necessary for survival. Understanding how these microscopic structures interact with their environment is crucial to comprehending biology at its most basic level. One of the most fundamental interactions is the movement of water across cell membranes, a process heavily influenced by the concentration of solutes in the surrounding solution. This article will explore the phenomenon of cell shrinkage, also known as plasmolysis, which occurs when cells are placed in solutions with a higher solute concentration than their internal environment.
Understanding Osmosis: The Driving Force Behind Cell Shrinkage
Osmosis is the passive movement of water across a selectively permeable membrane from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). This movement continues until equilibrium is reached, meaning the concentration of water is equal on both sides of the membrane. The selectively permeable membrane allows water to pass through but restricts the movement of solutes, creating the driving force for osmosis. Imagine a semi-permeable bag filled with pure water placed in a saltwater solution. Water will move out of the bag and into the salty solution until the concentration of water is the same on both sides, albeit with different solute concentrations.
The Role of Water Potential
Water potential is a measure of the tendency of water to move from one area to another. It's influenced by two main factors:
-
Solute potential: This refers to the effect of dissolved solutes on water potential. The more solutes present, the lower the solute potential, and the less likely water is to move into that area.
-
Pressure potential: This is the physical pressure exerted on the water. In plant cells, turgor pressure (the pressure of the cell contents against the cell wall) contributes to pressure potential.
Water always moves from an area of higher water potential to an area of lower water potential.
Hypertonic Solutions: The Shrinkage Scenario
A hypertonic solution is one that has a higher solute concentration than the solution inside the cell. When a cell is placed in a hypertonic solution, the water potential outside the cell is lower than the water potential inside the cell. This creates an osmotic gradient, causing water to move out of the cell and into the surrounding solution. This outward movement of water leads to a decrease in cell volume, resulting in cell shrinkage or plasmolysis.
Visualizing Plasmolysis: What Happens to the Cell?
The effects of plasmolysis are visually apparent, particularly in plant cells. As water leaves the cell, the cell membrane pulls away from the cell wall, a process known as plasmolysis. The cell becomes flaccid, losing its turgor pressure, and its shape can become distorted. In animal cells, which lack a rigid cell wall, the cell simply shrinks in size, becoming wrinkled and potentially leading to cell death if the water loss is significant.
Factors Affecting the Rate of Shrinkage
Several factors influence the rate at which a cell shrinks in a hypertonic solution:
-
Concentration gradient: The steeper the concentration gradient (the greater the difference in solute concentration between the cell and the solution), the faster water will move out of the cell, resulting in faster shrinkage.
-
Surface area to volume ratio: Cells with a higher surface area to volume ratio will generally shrink faster because there's more membrane area available for water to pass through. Smaller cells, therefore, tend to shrink faster than larger cells.
-
Membrane permeability: The permeability of the cell membrane to water also plays a role. More permeable membranes will facilitate faster water movement and faster shrinkage.
-
Temperature: Higher temperatures generally increase the rate of osmosis, thus increasing the rate of cell shrinkage.
Isotonic Solutions: The Balanced State
In contrast to a hypertonic solution, an isotonic solution has the same solute concentration as the solution inside the cell. In this scenario, there is no net movement of water across the cell membrane. The water potential is equal on both sides of the membrane, maintaining cell volume and shape. This is the ideal environment for many cells, allowing them to function optimally without the stress of water gain or loss.
Hypotonic Solutions: Cell Swelling and Lysis
A hypotonic solution has a lower solute concentration than the solution inside the cell. In this case, water moves into the cell, causing it to swell. Plant cells, with their rigid cell walls, can withstand this influx of water to a certain extent, becoming turgid. However, if the influx is excessive, the cell wall could potentially burst. Animal cells, lacking a protective cell wall, are more susceptible to cell lysis (bursting) when placed in a hypotonic solution.
The Significance of Osmosis in Biology
Osmosis is a fundamental process with far-reaching implications in various biological contexts:
-
Plant physiology: Osmosis is crucial for maintaining turgor pressure in plants, which is essential for plant growth, support, and the opening and closing of stomata (tiny pores on leaves that regulate gas exchange). Wilting occurs when plants lose turgor pressure due to water loss through osmosis.
-
Animal physiology: Osmosis plays a vital role in maintaining the balance of fluids and electrolytes in the body. The kidneys, for example, regulate the concentration of solutes in the blood through osmosis, ensuring proper hydration and electrolyte balance.
-
Cell transport: Many transport processes in cells are indirectly linked to osmosis, as the movement of water can influence the concentration gradients that drive other transport mechanisms.
-
Medical applications: Understanding osmosis is critical in various medical applications, such as intravenous fluid administration and the treatment of dehydration. The appropriate tonicity of intravenous fluids is vital to prevent cell damage.
Practical Applications and Real-World Examples
The principle of osmosis is not limited to theoretical discussions; it finds practical application in diverse fields:
-
Food preservation: Osmosis is employed in preserving foods like pickles and jams. High solute concentration in the brine or sugar solution draws water out of microorganisms, inhibiting their growth and preventing spoilage.
-
Water purification: Reverse osmosis is a technology used to purify water by forcing water through a semi-permeable membrane against the osmotic gradient. This removes impurities and salts, producing clean drinking water.
-
Agriculture: Understanding osmotic principles is important for irrigation practices. Providing plants with the right amount of water, neither too little nor too much, is essential for their health and yield.
Conclusion: Osmosis – A Crucial Biological Process
Cell shrinkage in hypertonic solutions, a consequence of osmosis, is a fundamental biological process with significant implications across diverse fields. Understanding the principles of osmosis, including the concepts of water potential, hypertonic, isotonic, and hypotonic solutions, is essential for comprehending cellular function, plant physiology, animal physiology, and various technological applications. The intricate balance of water movement across cell membranes is a testament to the elegance and precision of biological systems, and its study continues to yield valuable insights into the workings of life itself. Further research continues to unravel the complexities of osmotic processes and their influence on cellular behavior, ensuring continued advancements in various scientific and technological disciplines. This understanding is paramount not just for biological studies but for solving critical real-world problems ranging from food preservation to water purification and medical treatments.
Latest Posts
Latest Posts
-
42 Is What Percent Of 120
Mar 25, 2025
-
A Stone Is Thrown Vertically Upward
Mar 25, 2025
-
Carries Blood Away From The Kidney
Mar 25, 2025
-
Which Of The Following Is Not A Pyrimidine
Mar 25, 2025
-
The Specific Amino Acid Sequence In A Protein Is Its
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Cells Shrink When They Are Placed In Solutions That Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
