Cells In A Hypertonic Solution Will
News Leon
Mar 17, 2025 · 6 min read
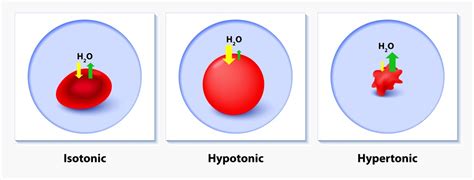
Table of Contents
Cells in a Hypertonic Solution Will: A Deep Dive into Osmosis and Cellular Responses
Understanding how cells behave in different environments is fundamental to biology. One crucial concept is osmosis, the movement of water across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement is driven by the difference in water potential, ultimately aiming to achieve equilibrium. A key scenario to consider is what happens when a cell is placed in a hypertonic solution. This article will explore this in detail, examining the cellular mechanisms involved and the consequences for different cell types.
What is a Hypertonic Solution?
Before delving into the cellular effects, let's define a hypertonic solution. A hypertonic solution is one in which the concentration of solutes (dissolved substances) is higher outside the cell than inside the cell. Conversely, the concentration of water is lower outside the cell compared to inside. This difference in solute concentration creates a water potential gradient, initiating the movement of water.
Osmosis: The Driving Force
Osmosis is the passive transport of water across a selectively permeable membrane. This membrane allows the passage of water molecules but restricts the movement of many solutes. The driving force behind osmosis is the tendency of water to move from an area of high water potential (low solute concentration) to an area of low water potential (high solute concentration). This movement continues until the water potential is equal on both sides of the membrane, or until a counteracting force is established.
Cellular Response in a Hypertonic Solution
When a cell is placed in a hypertonic solution, water moves out of the cell, down its concentration gradient, and into the surrounding solution. This outflow of water causes the cell to lose volume and shrink. The extent of shrinkage depends on several factors, including the initial cell volume, the solute concentration gradient, and the permeability of the cell membrane.
Animal Cells: Crenation
Animal cells, lacking a rigid cell wall, are particularly susceptible to the effects of a hypertonic environment. The loss of water leads to crenation, a process where the cell shrinks and becomes wrinkled or scalloped in appearance. Severe crenation can damage the cell membrane, disrupting cellular function and potentially leading to cell death. The delicate balance of internal pressure and membrane integrity is significantly compromised.
Plant Cells: Plasmolysis
Plant cells, possessing a rigid cell wall, respond differently to a hypertonic environment. Initially, water loss occurs, just as in animal cells. However, the cell wall provides structural support, preventing the complete collapse of the cell. The process of water loss and shrinkage in plant cells is known as plasmolysis.
During plasmolysis, the plasma membrane pulls away from the cell wall, a phenomenon called protoplast shrinkage. This separation creates gaps between the membrane and the cell wall. While the cell wall maintains its shape, the internal pressure (turgor pressure) significantly decreases. The extent of plasmolysis depends on the severity and duration of the hypertonic stress. Severe plasmolysis can disrupt cellular processes, affecting nutrient transport and ultimately impacting the plant's health and survival.
Bacterial Cells: Plasmolysis (and other considerations)
Similar to plant cells, bacterial cells also exhibit plasmolysis in a hypertonic environment. Their rigid cell wall prevents complete collapse, but water loss shrinks the cytoplasm, causing the plasma membrane to detach from the cell wall. This process can inhibit growth and reproduction, potentially leading to bacterial dormancy or death. However, some bacteria possess mechanisms to cope with hypertonic stress, such as accumulating compatible solutes within the cytoplasm to increase their internal osmotic pressure, partially counteracting the water loss.
The Role of Aquaporins
Aquaporins are integral membrane proteins that form channels specifically for water molecules. They facilitate the rapid passage of water across the cell membrane, significantly influencing the rate of osmosis. The number and activity of aquaporins can vary between cell types and can be regulated in response to environmental changes. In a hypertonic environment, the activity of aquaporins might be modulated to control the rate of water efflux, minimizing the impact of water loss.
Cellular Mechanisms to Cope with Hypertonicity
Cells have evolved various mechanisms to cope with hypertonic stress and maintain their internal balance:
1. Compatible Solutes Accumulation:
Many organisms accumulate compatible solutes within their cytoplasm. These are small organic molecules that do not interfere with cellular processes even at high concentrations. By increasing the internal solute concentration, these solutes help to reduce the water potential gradient, mitigating the effects of water loss. Examples include proline, glycine betaine, and trehalose.
2. Ion Channels and Pumps:
Cells can regulate the influx and efflux of ions to adjust their internal osmotic pressure. Ion channels and pumps, such as the sodium-potassium pump, actively transport ions across the membrane, contributing to maintaining osmotic balance.
3. Osmoprotectants:
Osmoprotectants are specialized molecules that protect cellular components from the damaging effects of high solute concentrations. They stabilize proteins and membranes, preventing denaturation and disruption of cellular functions during hypertonic stress.
4. Changes in Gene Expression:
Cells can respond to hypertonic stress by altering the expression of genes involved in stress response. This can lead to the increased production of compatible solutes, chaperone proteins (protecting other proteins from damage), and other molecules that help the cell to adapt to the hypertonic environment.
Consequences of Prolonged Hypertonic Stress
Prolonged exposure to a hypertonic environment can have severe consequences for cells. Continuous water loss can lead to irreversible damage to cellular structures and ultimately cell death. The disruption of cellular processes, including metabolism, protein synthesis, and DNA replication, can severely compromise cellular function. This can have cascading effects on tissues and organs, impacting the overall health of the organism.
Examples of Hypertonic Environments
Understanding hypertonic solutions is crucial for a variety of contexts. For instance, high salinity environments, such as saltwater bodies, represent hypertonic conditions for many freshwater organisms. Likewise, certain medical treatments utilize hypertonic solutions to create specific osmotic effects.
Conclusion
The behavior of cells in a hypertonic solution highlights the importance of osmosis and the crucial role of water balance in maintaining cellular function. The responses of different cell types, from the crenation of animal cells to the plasmolysis of plant and bacterial cells, demonstrate the diversity of cellular adaptations to environmental challenges. The ability of cells to cope with hypertonicity through various mechanisms underscores the remarkable adaptability of life. Understanding these processes is vital for various fields, including medicine, agriculture, and environmental science, enabling us to address challenges related to cellular health and survival in diverse environments. Further research continues to unravel the intricate details of cellular responses to osmotic stress and identify novel strategies for mitigating the negative consequences of hypertonic conditions. This ongoing research is crucial for advancing our understanding of fundamental biological processes and developing innovative solutions for practical applications.
Latest Posts
Latest Posts
-
How Many Oxygen Molecules Can One Hemoglobin Carry
Mar 18, 2025
-
Which Of The Following Is Not A Form Of Precipitation
Mar 18, 2025
-
Which Statement About Natural Selection Is True
Mar 18, 2025
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Cells In A Hypertonic Solution Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
