Can Ammonia Be Decomposed By A Chemical Change
News Leon
Mar 30, 2025 · 7 min read
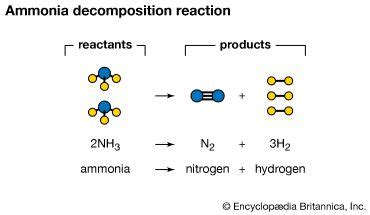
Table of Contents
Can Ammonia Be Decomposed by a Chemical Change?
Ammonia, a simple yet crucial compound with the chemical formula NH₃, plays a vital role in various industrial processes and natural cycles. Understanding its chemical behavior, particularly its susceptibility to decomposition, is essential for various applications, from fertilizer production to environmental studies. This comprehensive article will explore the decomposition of ammonia through chemical changes, delving into the methods, conditions, and underlying principles involved. We'll examine the factors influencing the decomposition process, its practical applications, and safety considerations.
Understanding Ammonia's Chemical Structure and Stability
Before delving into decomposition methods, it's crucial to grasp ammonia's inherent chemical structure and stability. Ammonia is a covalent compound, meaning its atoms are held together by shared electrons. The nitrogen atom shares three electron pairs with three hydrogen atoms, resulting in a trigonal pyramidal geometry. This structure, while relatively stable under normal conditions, is not immutable. The nitrogen-hydrogen bonds, while strong, can be broken under specific circumstances, leading to decomposition.
The Nitrogen-Hydrogen Bond Strength: A Key Factor
The strength of the nitrogen-hydrogen bonds directly influences ammonia's stability and decomposition. While relatively strong compared to some other covalent bonds, they are not unbreakable. The bond energy needed to break these bonds is significant, requiring substantial energy input to initiate the decomposition process. This is why ammonia is relatively stable under ambient conditions.
Chemical Methods for Decomposing Ammonia
Several chemical methods can be employed to decompose ammonia, each relying on specific conditions and catalysts to overcome the energy barrier associated with breaking the N-H bonds.
1. Thermal Decomposition: Heat as the Driving Force
Thermal decomposition involves applying high temperatures to break the N-H bonds. This is a common method, but it requires extremely high temperatures, typically above 400°C, to achieve significant decomposition. The reaction proceeds as follows:
2NH₃(g) ⇌ N₂(g) + 3H₂(g)
This reaction is endothermic, meaning it absorbs heat. The equilibrium shifts towards decomposition at higher temperatures. The presence of catalysts can significantly lower the activation energy required for this reaction, making the decomposition process more efficient at lower temperatures.
Catalyst Impact on Thermal Decomposition
The use of catalysts is pivotal in accelerating thermal decomposition. Various metals and metal oxides, including iron, nickel, and platinum, act as effective catalysts. These catalysts provide an alternative reaction pathway with a lower activation energy, promoting faster decomposition at lower temperatures. The exact mechanism of catalysis involves adsorption of ammonia onto the catalyst surface, weakening the N-H bonds and facilitating their cleavage.
2. Catalytic Decomposition: Enhancing Reaction Rates
As mentioned, catalysts play a crucial role in enhancing ammonia decomposition rates. They provide a surface for the ammonia molecules to adsorb onto, thereby weakening the N-H bonds and lowering the activation energy required for decomposition. This method is often preferred over simple thermal decomposition because it leads to faster and more efficient decomposition at lower temperatures, making it energy-efficient and practical.
Choosing the Right Catalyst: A Matter of Efficiency
The choice of catalyst depends on various factors, including the desired reaction rate, temperature, and cost. Different catalysts exhibit varying levels of activity and selectivity. Optimizing the catalyst choice is critical for achieving high efficiency in ammonia decomposition.
3. Electrochemical Decomposition: Utilizing Electrical Energy
Electrochemical decomposition, also known as electrolysis, involves using an electric current to decompose ammonia. This method typically involves passing an electric current through an aqueous solution of ammonia. The electric current provides the energy necessary to break the N-H bonds, resulting in the formation of nitrogen and hydrogen gases.
Electrode Materials and Efficiency
The efficiency of electrochemical decomposition depends on several factors, including the electrode materials used, the electrolyte composition, and the applied current density. Selection of appropriate electrode materials that resist corrosion and promote efficient electron transfer is critical for optimal performance.
4. Photochemical Decomposition: Light-Driven Decomposition
Photochemical decomposition uses light energy to initiate the decomposition of ammonia. This method requires specific wavelengths of light, typically ultraviolet (UV) light, to excite the ammonia molecules and provide the energy needed to break the N-H bonds.
Role of UV Light and Sensitivity
UV light provides the energy to excite ammonia molecules to a higher energy state, weakening the N-H bonds and making them susceptible to cleavage. The process is sensitive to the intensity and wavelength of the UV light used.
Factors Influencing Ammonia Decomposition
Several factors influence the rate and extent of ammonia decomposition. Understanding these factors is critical for controlling and optimizing the decomposition process.
Temperature: A Primary Influence
Temperature plays a crucial role in determining the rate of decomposition. Higher temperatures generally lead to faster decomposition rates, as they provide the necessary energy to overcome the activation energy barrier.
Pressure: Impact on Equilibrium
Pressure also affects the equilibrium of the decomposition reaction. Increasing the pressure shifts the equilibrium towards the reactants (ammonia), reducing the extent of decomposition. Conversely, decreasing the pressure favors the products (nitrogen and hydrogen).
Catalyst Concentration: Enhancing Reaction Rates
The concentration of the catalyst significantly influences the rate of catalytic decomposition. Higher catalyst concentrations generally lead to faster decomposition rates, as more active sites are available for ammonia adsorption and decomposition.
Surface Area of Catalyst: Maximizing Efficiency
The surface area of the catalyst is another important factor. A larger surface area provides more active sites for the reaction, leading to enhanced efficiency. Catalysts with high surface area, like finely divided powders, are often preferred for ammonia decomposition.
Applications of Ammonia Decomposition
The decomposition of ammonia finds applications in several industrial processes and research areas.
1. Hydrogen Production: A Clean Energy Source
One major application is hydrogen production. The decomposition of ammonia into nitrogen and hydrogen is a promising method for producing clean hydrogen fuel, which can be used in fuel cells to generate electricity.
2. Nitrogen Fixation: Mimicking Nature
Ammonia decomposition studies are crucial in understanding nitrogen fixation, a natural process by which atmospheric nitrogen is converted into ammonia. Understanding the decomposition process offers insights into reversing this natural cycle and its implications for the environment.
3. Industrial Chemical Synthesis: Utilizing Byproducts
The nitrogen and hydrogen generated from ammonia decomposition are valuable industrial chemicals. They are used as building blocks in various chemical synthesis processes.
4. Research and Development: Exploring New Catalysts
Research into ammonia decomposition continues to explore the development of more efficient and cost-effective catalysts for industrial processes. This research includes studies on novel catalyst materials, reaction mechanisms, and optimization strategies.
Safety Considerations in Ammonia Decomposition
Ammonia decomposition, particularly thermal decomposition, can generate high temperatures and pressures. Therefore, adequate safety measures are essential to prevent accidents and ensure safe operation.
Temperature Control: Preventing Runaway Reactions
Careful temperature control is critical to prevent runaway reactions. Overheating can lead to uncontrolled decomposition and potentially hazardous conditions. Appropriate safety devices, such as pressure relief valves and temperature sensors, are necessary to maintain safe operating temperatures.
Pressure Management: Handling Gases
Effective pressure management is vital, especially during high-temperature decomposition. Appropriate containment vessels and pressure-relief systems are required to prevent pressure buildup and potential explosions.
Handling Gases: Safety Precautions
Proper handling of the gases produced during decomposition, such as nitrogen and hydrogen, is essential. Nitrogen is generally inert, but hydrogen is highly flammable and requires careful handling to prevent fire hazards.
Conclusion: Ammonia Decomposition—A Versatile Process
Ammonia decomposition via chemical changes is a versatile process with significant applications in various industries and research areas. Understanding the underlying chemistry, influencing factors, and safety considerations is crucial for optimizing the decomposition process and utilizing its products effectively. The development of efficient and cost-effective methods for ammonia decomposition continues to be an area of active research, promising further advancements in clean energy production, industrial chemical synthesis, and environmental technologies. The ongoing investigation into improved catalysts and optimization techniques will undoubtedly lead to even more impactful applications of ammonia decomposition in the future. The versatility of this process makes it a valuable tool for scientists and engineers striving to address global energy and environmental challenges.
Latest Posts
Latest Posts
-
Coordination Number Of Hexagonal Close Packing
Apr 01, 2025
-
What Goes Up And Downstairs Without Moving
Apr 01, 2025
-
What Is The Main Difference Between
Apr 01, 2025
-
Which Of The Following Forms A Molecular Solid
Apr 01, 2025
-
What Is The Least Electronegative Element
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Can Ammonia Be Decomposed By A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
