Boyle's Charles And Gay Lussac's Gas Problems
News Leon
Mar 26, 2025 · 7 min read
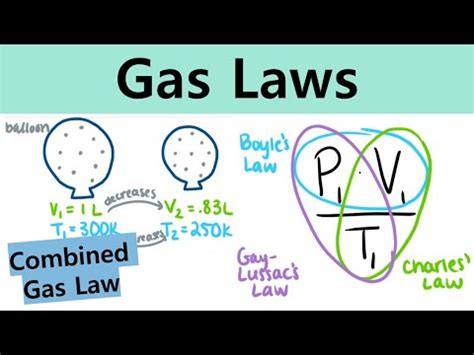
Table of Contents
Boyle's, Charles', and Gay-Lussac's Gas Laws: A Comprehensive Guide to Solving Problems
Understanding the behavior of gases is crucial in various scientific fields, from chemistry and physics to engineering and meteorology. Three fundamental gas laws – Boyle's Law, Charles's Law, and Gay-Lussac's Law – provide a framework for comprehending how pressure, volume, and temperature affect gases. This comprehensive guide delves into each law, explaining their principles, demonstrating problem-solving techniques, and exploring their combined application in more complex scenarios.
Boyle's Law: The Inverse Relationship Between Pressure and Volume
Boyle's Law, discovered by Robert Boyle in 1662, states that the pressure of a gas is inversely proportional to its volume when the temperature and the amount of gas are held constant. This means that if you increase the pressure on a gas, its volume will decrease proportionally, and vice versa. Mathematically, this relationship is expressed as:
P₁V₁ = P₂V₂
Where:
- P₁ is the initial pressure
- V₁ is the initial volume
- P₂ is the final pressure
- V₂ is the final volume
Understanding the Underlying Principle: At a constant temperature, gas particles are constantly moving and colliding with each other and the walls of their container. Increasing the pressure means more collisions per unit area, effectively compressing the gas and reducing its volume. Conversely, reducing the pressure allows the gas particles more space to move, increasing the volume.
Problem-Solving Example:
A gas occupies a volume of 5.0 L at a pressure of 1.0 atm. What will be its volume if the pressure is increased to 2.5 atm at constant temperature?
Using Boyle's Law:
P₁V₁ = P₂V₂
(1.0 atm)(5.0 L) = (2.5 atm)(V₂)
V₂ = (1.0 atm * 5.0 L) / 2.5 atm = 2.0 L
Therefore, the volume will decrease to 2.0 L when the pressure is increased to 2.5 atm.
Charles's Law: The Direct Relationship Between Volume and Temperature
Charles's Law, attributed to Jacques Charles in the late 18th century, describes the relationship between the volume and temperature of a gas when the pressure and the amount of gas remain constant. It states that the volume of a gas is directly proportional to its absolute temperature. This means that if you increase the temperature of a gas, its volume will increase proportionally, and vice versa. The equation for Charles's Law is:
V₁/T₁ = V₂/T₂
Where:
- V₁ is the initial volume
- T₁ is the initial absolute temperature (in Kelvin)
- V₂ is the final volume
- T₂ is the final absolute temperature (in Kelvin)
Crucial Note: Temperature in gas law calculations must always be expressed in Kelvin (K). To convert from Celsius (°C) to Kelvin, use the formula: K = °C + 273.15
Understanding the Underlying Principle: Increasing the temperature increases the kinetic energy of the gas particles, causing them to move faster and collide more forcefully with the container walls. This increased kinetic energy leads to an expansion of the gas volume.
Problem-Solving Example:
A balloon has a volume of 2.5 L at 25°C. What will be its volume if the temperature is increased to 50°C at constant pressure?
First, convert temperatures to Kelvin:
T₁ = 25°C + 273.15 = 298.15 K T₂ = 50°C + 273.15 = 323.15 K
Using Charles's Law:
V₁/T₁ = V₂/T₂
(2.5 L) / (298.15 K) = V₂ / (323.15 K)
V₂ = (2.5 L * 323.15 K) / 298.15 K ≈ 2.71 L
The balloon's volume will increase to approximately 2.71 L.
Gay-Lussac's Law: The Direct Relationship Between Pressure and Temperature
Gay-Lussac's Law, named after Joseph Louis Gay-Lussac, explains the relationship between the pressure and temperature of a gas when the volume and the amount of gas remain constant. It states that the pressure of a gas is directly proportional to its absolute temperature. This means that if you increase the temperature of a gas in a fixed volume, its pressure will increase proportionally. The equation is:
P₁/T₁ = P₂/T₂
Where:
- P₁ is the initial pressure
- T₁ is the initial absolute temperature (in Kelvin)
- P₂ is the final pressure
- T₂ is the final absolute temperature (in Kelvin)
Understanding the Underlying Principle: As temperature increases, gas particles move faster, resulting in more frequent and forceful collisions with the container walls. This increased collision rate translates to a higher pressure.
Problem-Solving Example:
A pressure cooker contains steam at a pressure of 1.5 atm at 100°C. If the temperature is raised to 120°C while the volume remains constant, what will be the new pressure?
First, convert temperatures to Kelvin:
T₁ = 100°C + 273.15 = 373.15 K T₂ = 120°C + 273.15 = 393.15 K
Using Gay-Lussac's Law:
P₁/T₁ = P₂/T₂
(1.5 atm) / (373.15 K) = P₂ / (393.15 K)
P₂ = (1.5 atm * 393.15 K) / 373.15 K ≈ 1.58 atm
The pressure will increase to approximately 1.58 atm.
Combining the Gas Laws: The Combined Gas Law
Often, situations involve changes in all three variables – pressure, volume, and temperature. In such cases, the Combined Gas Law is used, which combines Boyle's, Charles's, and Gay-Lussac's laws:
(P₁V₁)/T₁ = (P₂V₂)/T₂
This equation allows you to solve problems where any two of the three variables change while the amount of gas remains constant.
Problem-Solving Example:
A gas sample has a volume of 3.0 L at a pressure of 1.2 atm and a temperature of 27°C. What will be its volume if the pressure is increased to 1.8 atm and the temperature is decreased to 17°C?
First, convert temperatures to Kelvin:
T₁ = 27°C + 273.15 = 300.15 K T₂ = 17°C + 273.15 = 290.15 K
Using the Combined Gas Law:
(P₁V₁)/T₁ = (P₂V₂)/T₂
(1.2 atm * 3.0 L) / 300.15 K = (1.8 atm * V₂) / 290.15 K
V₂ = (1.2 atm * 3.0 L * 290.15 K) / (300.15 K * 1.8 atm) ≈ 1.74 L
The volume will decrease to approximately 1.74 L.
The Ideal Gas Law: A More Comprehensive Approach
While Boyle's, Charles's, and Gay-Lussac's laws are valuable for understanding basic gas behavior, they assume ideal conditions. The Ideal Gas Law provides a more accurate description of gas behavior, especially at higher temperatures and lower pressures:
PV = nRT
Where:
- P is the pressure
- V is the volume
- n is the number of moles of gas
- R is the ideal gas constant (0.0821 L·atm/mol·K)
- T is the absolute temperature (in Kelvin)
The Ideal Gas Law accounts for the amount of gas present, making it suitable for a wider range of conditions. Solving problems using the Ideal Gas Law often involves converting between mass, moles, and molar mass.
Problem-Solving Example:
What is the pressure exerted by 2.0 moles of an ideal gas in a 5.0 L container at 25°C?
First, convert temperature to Kelvin:
T = 25°C + 273.15 = 298.15 K
Using the Ideal Gas Law:
PV = nRT
P * 5.0 L = 2.0 mol * 0.0821 L·atm/mol·K * 298.15 K
P = (2.0 mol * 0.0821 L·atm/mol·K * 298.15 K) / 5.0 L ≈ 9.77 atm
The pressure exerted by the gas is approximately 9.77 atm.
Conclusion: Mastering Gas Law Calculations
Understanding and applying Boyle's, Charles's, Gay-Lussac's, and the combined and ideal gas laws are essential for anyone studying chemistry, physics, or related fields. By mastering the principles and practicing problem-solving techniques, you can gain a strong grasp of gas behavior and its implications in various scientific and engineering applications. Remember to always convert temperatures to Kelvin and to choose the appropriate gas law based on the variables that remain constant or change in the given problem. Consistent practice and a clear understanding of the underlying principles will pave the way for success in solving even complex gas law problems. This knowledge is not just theoretical; it is foundational to many real-world applications, from designing efficient engines to understanding weather patterns.
Latest Posts
Latest Posts
-
Which Change To Earth Occurs Fastest
Mar 29, 2025
-
How Many Seconds In Two Days
Mar 29, 2025
-
What Is A Nation State Class 10
Mar 29, 2025
-
Do Metals Form Anions Or Cations
Mar 29, 2025
-
The Cop And The Anthem Summary
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Boyle's Charles And Gay Lussac's Gas Problems . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
