Bond Order Of No In No3
News Leon
Mar 17, 2025 · 5 min read
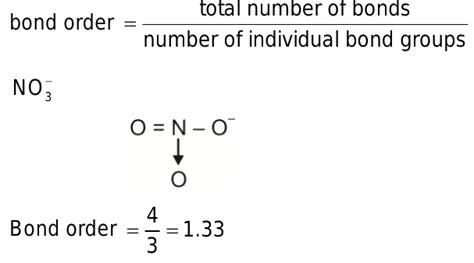
Table of Contents
Delving into the Bond Order of N-O in NO₃⁻: A Comprehensive Analysis
The nitrate ion (NO₃⁻) is a ubiquitous species in chemistry, featuring prominently in various fields, from fertilizers to explosives. Understanding its structure, particularly the bond order of the nitrogen-oxygen bonds, is crucial to grasping its reactivity and properties. This article provides a comprehensive exploration of the bond order of N-O bonds in the NO₃⁻ ion, moving beyond simplistic explanations to delve into the complexities of resonance structures and molecular orbital theory.
Understanding Resonance Structures in NO₃⁻
The nitrate ion displays resonance, a phenomenon where a single Lewis structure is insufficient to represent the molecule's true bonding. Instead, several resonance structures contribute to the overall structure, representing a hybrid. Let's examine these structures:
The Three Equivalent Resonance Structures
Nitrate ion's Lewis structures depict a central nitrogen atom surrounded by three oxygen atoms. One oxygen atom forms a double bond with the nitrogen, while the other two form single bonds. However, the actual structure isn't static; the double bond is delocalized across all three nitrogen-oxygen bonds. We can represent this using three equivalent resonance structures:
(Insert image here: Three resonance structures of NO₃⁻, showing the double bond shifting between oxygen atoms)
Each resonance structure shows a formal charge distribution, crucial in understanding the stability of the ion. Note that the formal charge doesn't represent the actual charge on each atom but is a bookkeeping tool to understand electron distribution within the molecule.
The Resonance Hybrid: A More Accurate Representation
The true representation of the nitrate ion is not any single Lewis structure but a resonance hybrid. This hybrid is a weighted average of the contributing resonance structures. In the case of NO₃⁻, all three resonance structures contribute equally, leading to a symmetrical structure. This symmetry is reflected in the experimental observation of equal bond lengths between the nitrogen and oxygen atoms.
Calculating the Bond Order
The concept of bond order is vital for understanding the strength and stability of a bond. It represents the number of chemical bonds between a pair of atoms. In simple diatomic molecules, bond order is easily calculated, but in polyatomic ions with resonance, it becomes a weighted average across the resonance structures.
Bond Order Calculation Using Resonance Structures
For the nitrate ion:
-
Identify the total number of N-O bonds: In each resonance structure, there are three N-O bonds.
-
Total number of bonding electrons: Considering each resonance structure, the total number of N-O bonds across the three structures is 3 + 1 + 1 + 1 + 1 + 1 = 4.
-
Total number of resonance structures: We have three equivalent resonance structures.
-
Bond order calculation: The total number of bonds (4) is distributed equally among the three N-O bonds, leading to a bond order of (4/3) ≈ 1.33.
Implications of the Bond Order: Bond Length and Strength
The bond order of 1.33 reflects the intermediate nature of the N-O bonds in NO₃⁻. The bond length and strength are intermediate between a single bond (bond order 1) and a double bond (bond order 2). Experimentally observed N-O bond lengths in NO₃⁻ corroborate this. This intermediate bond order reflects the delocalized nature of the electrons.
Beyond Resonance: Molecular Orbital Theory (MOT) Perspective
While resonance structures provide a valuable visual representation, molecular orbital theory offers a more rigorous approach to understanding the bonding in NO₃⁻.
Constructing Molecular Orbitals
In MOT, atomic orbitals combine to form molecular orbitals spanning the entire molecule. In NO₃⁻, the nitrogen's 2s and 2p orbitals, along with the oxygen's 2s and 2p orbitals, combine to form a set of bonding and antibonding molecular orbitals.
Delocalization and Bonding Orbitals
The key to understanding the bond order in the MOT picture lies in the delocalization of electrons across the bonding molecular orbitals that extend across all three N-O bonds. These delocalized electrons result in the observed equal bond lengths and intermediate bond strength.
Correlation with Bond Order from Resonance
The results obtained from the MOT analysis are consistent with the bond order calculated from resonance structures. The delocalization predicted by MOT leads to the fractional bond order, mirroring the 1.33 bond order from the resonance approach.
The Significance of the Bond Order of N-O in NO₃⁻
The 1.33 bond order in the nitrate ion has several significant implications:
Reactivity and Stability
The intermediate strength of the N-O bonds influences the ion's reactivity. The bonds are strong enough to provide stability, but they are also susceptible to breaking under certain conditions, making the nitrate ion a versatile reactant in various chemical processes.
Spectroscopic Properties
The bond order and electron delocalization significantly influence the spectroscopic properties of the nitrate ion. This influences its UV-Vis absorption spectrum, vibrational frequencies, and other spectroscopic parameters.
Applications in Chemistry and Beyond
The properties of the nitrate ion are inextricably linked to its bond order, which explains the diverse applications of nitrate salts and the nitrate ion itself, spanning from fertilizers in agriculture to crucial roles in various chemical and biological processes.
Advanced Considerations and Further Exploration
While the 1.33 bond order is a good approximation, it is important to remember that this is an average. Subtle variations in bond lengths can exist due to vibrational motion and subtle environmental factors.
Furthermore, sophisticated computational techniques, such as density functional theory (DFT) calculations, can provide a more precise picture of the electron distribution and bond order in NO₃⁻, potentially refining the 1.33 value further. These methods can also account for electron correlation, a crucial factor not explicitly considered in simpler models.
Conclusion
The bond order of N-O in NO₃⁻ is not simply 1 or 2 but a fractional value of approximately 1.33, a testament to the power of resonance and the more nuanced picture offered by molecular orbital theory. This intermediate bond order significantly influences the ion's stability, reactivity, and spectroscopic properties, explaining its wide-ranging applications in various fields. Understanding this bond order is crucial to grasping the chemistry of the nitrate ion and its profound impact across different scientific disciplines. Further exploration using advanced computational techniques can provide even greater insights into the intricacies of bonding within this important chemical species.
Latest Posts
Latest Posts
-
Dover Beach By Matthew Arnold Explanation
Mar 18, 2025
-
How Many Oxygen Molecules Can One Hemoglobin Carry
Mar 18, 2025
-
Which Of The Following Is Not A Form Of Precipitation
Mar 18, 2025
-
Which Statement About Natural Selection Is True
Mar 18, 2025
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Bond Order Of No In No3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
