Boiling Water Is A Chemical Or Physical Change
News Leon
Mar 18, 2025 · 5 min read
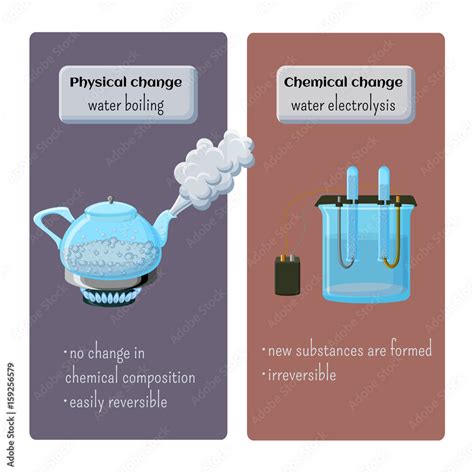
Table of Contents
Is Boiling Water a Chemical or Physical Change? A Deep Dive
The question of whether boiling water represents a chemical or physical change is a deceptively simple one that often sparks debate. While it might seem obvious at first glance, a deeper understanding of the concepts involved reveals a more nuanced answer. This article will delve into the intricacies of chemical and physical changes, explore the process of boiling water in detail, and ultimately provide a definitive answer supported by scientific evidence. We'll also discuss the implications of this classification and explore related concepts to provide a comprehensive understanding of this fundamental scientific principle.
Understanding Chemical vs. Physical Changes
Before we examine the boiling of water, it’s crucial to establish a clear understanding of the defining characteristics of chemical and physical changes.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same at a molecular level. Examples of physical changes include:
- Melting ice: Ice (solid water) changes to liquid water, but the water molecules remain H₂O.
- Boiling water: Liquid water changes to water vapor (steam), but the molecules remain H₂O.
- Dissolving sugar in water: The sugar molecules disperse in the water, but their chemical structure doesn't change. They can be recovered by evaporation.
- Crushing a can: The can's shape changes, but the metal remains the same.
The key takeaway here is that physical changes are reversible, at least in theory. You can refreeze liquid water into ice, condense steam back into liquid water, and recover the sugar from the solution.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into new substances with different chemical properties. This transformation occurs at the molecular level, involving the breaking and formation of chemical bonds. Examples of chemical changes include:
- Burning wood: Wood reacts with oxygen to produce ash, carbon dioxide, and water. The original wood is gone, replaced by entirely different substances.
- Rusting iron: Iron reacts with oxygen and water to form iron oxide (rust), a new substance with different properties.
- Baking a cake: The ingredients undergo a series of chemical reactions, creating a new substance with different properties than the original ingredients.
- Digestion: Food is broken down into simpler molecules through a series of chemical reactions.
Chemical changes are generally irreversible without further chemical intervention. You cannot easily turn ash back into wood, or rust back into iron.
Analyzing the Boiling of Water
Now, let's apply this understanding to the specific case of boiling water. When water boils, it transitions from its liquid state to its gaseous state (steam). The molecules are moving faster due to the increased temperature and overcome the intermolecular forces holding them together in the liquid phase. This leads to the formation of water vapor. Crucially, the chemical composition of the water remains unchanged. The water molecules remain H₂O.
No new chemical substances are formed during the boiling process. The only change is the physical state of the water. If you were to condense the steam back into liquid water, you would recover the same water molecules you started with. This reversibility is a hallmark of a physical change.
The Role of Temperature and Energy
The process of boiling water involves a significant energy input – the heat energy is needed to overcome the intermolecular forces within the liquid water and transition it into the gaseous phase. This energy increase increases the kinetic energy of the water molecules, causing them to move faster and further apart. However, this energy input does not alter the chemical bonds within the water molecule itself.
Considering Impurities
It's important to note that if the water contains dissolved impurities, boiling might lead to some subtle changes in concentration. However, these are changes in the concentration of the impurities, not a fundamental alteration of the water molecules themselves. The boiling process itself remains a physical change. Even if some impurities decompose at high temperatures, this is a separate chemical change affecting the impurities, not the water itself.
The Definitive Answer: Boiling Water is a Physical Change
Based on the analysis above, the definitive answer is that boiling water is a physical change. The chemical composition of the water remains unaltered throughout the process. The only change is the physical state of the water, from liquid to gas. This change is reversible, as evidenced by the condensation of steam back into liquid water.
Implications and Related Concepts
Understanding the distinction between chemical and physical changes is fundamental to many scientific disciplines, including chemistry, physics, and materials science. This understanding has practical implications across various fields.
For example:
- Cooking: Many cooking processes involve both physical and chemical changes. Boiling vegetables is primarily a physical change (water evaporates), while baking a cake involves numerous chemical changes (interactions between ingredients).
- Industrial Processes: Many industrial processes, such as distillation and evaporation, rely on the principles of physical changes to separate and purify substances.
- Environmental Science: Understanding the physical and chemical changes occurring in the environment is essential for addressing environmental challenges such as pollution and climate change.
Conclusion
The simple act of boiling water offers a surprisingly rich opportunity to explore the fundamental concepts of chemical and physical changes. While the initial intuition might suggest a chemical reaction, a closer examination reveals that boiling water is a purely physical change. The water molecules remain intact, only their state of aggregation changes. This distinction underscores the importance of understanding the molecular-level processes involved in physical and chemical transformations. The ability to distinguish between these two types of changes is fundamental to a deep understanding of chemistry and the world around us. Through careful observation and scientific analysis, we can appreciate the elegance and simplicity of a seemingly mundane phenomenon like boiling water.
Latest Posts
Latest Posts
-
Why Is Boiling Water A Physical Change
Mar 19, 2025
-
What Is The Product Of The Following Sequence Of Reactions
Mar 19, 2025
-
How Are Weathering And Erosion Difference
Mar 19, 2025
-
Double Replacement Examples In Real Life
Mar 19, 2025
-
The Study Of Population Is Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Is A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
