Benedict's Reagent Tests For The Presence Of
News Leon
Mar 20, 2025 · 6 min read
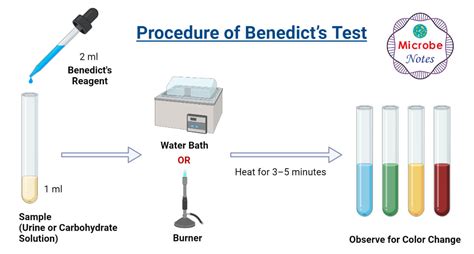
Table of Contents
Benedict's Reagent Test: A Comprehensive Guide to Detecting Reducing Sugars
Benedict's reagent is a widely used chemical reagent in biochemistry laboratories to detect the presence of reducing sugars. This article will delve into the intricacies of this test, explaining its mechanism, procedure, interpretation of results, and limitations. We'll also explore its applications and compare it to other similar tests. Understanding Benedict's test is crucial for anyone working with carbohydrates or conducting experiments involving sugar detection.
Understanding Reducing Sugars
Before diving into the specifics of the Benedict's test, let's establish a foundational understanding of reducing sugars. Reducing sugars are carbohydrates that possess a free aldehyde (-CHO) or ketone (-C=O) group. These groups are crucial because they're responsible for the reducing properties of these sugars. They can readily donate electrons to another molecule, specifically oxidizing themselves in the process.
Common examples of reducing sugars include:
- Glucose: A simple sugar found abundantly in fruits and blood.
- Fructose: Another simple sugar found in fruits and honey.
- Galactose: A simple sugar found in milk.
- Lactose: A disaccharide composed of glucose and galactose, found in milk.
- Maltose: A disaccharide composed of two glucose molecules, formed during starch digestion.
Non-reducing sugars, on the other hand, lack free aldehyde or ketone groups. Sucrose (table sugar) is a classic example. Because their anomeric carbon atoms are involved in a glycosidic bond, they cannot be oxidized by Benedict's reagent.
The Chemistry Behind Benedict's Reagent
Benedict's reagent is an alkaline solution of copper(II) sulfate, sodium citrate, and sodium carbonate. The key component is copper(II) sulfate, which provides the copper ions (Cu²⁺) needed for the reaction. Sodium citrate acts as a chelating agent, preventing the precipitation of copper(II) hydroxide. Sodium carbonate maintains the alkaline pH necessary for the reaction to proceed.
The reaction between a reducing sugar and Benedict's reagent is an oxidation-reduction reaction. The reducing sugar, containing a free aldehyde or ketone group, gets oxidized, donating electrons to the copper(II) ions (Cu²⁺). These copper(II) ions are reduced to copper(I) ions (Cu⁺), which then form a brick-red precipitate of copper(I) oxide (Cu₂O). The intensity of the brick-red precipitate is directly proportional to the concentration of the reducing sugar present in the sample.
Performing the Benedict's Test: A Step-by-Step Guide
The Benedict's test is relatively simple to perform, requiring minimal equipment and materials. Here's a detailed procedure:
-
Prepare the Sample: Prepare a solution of the substance you want to test. Dissolve a small amount of the sample in distilled water. The concentration of the sample will influence the intensity of the result; dilute samples may yield weaker positive results.
-
Add Benedict's Reagent: Add a few milliliters of Benedict's reagent to the sample solution. The ratio of sample to reagent can vary; refer to the specific instructions provided with the reagent.
-
Heat the Mixture: Gently heat the mixture in a boiling water bath for 3-5 minutes. Do not boil the mixture directly, as this could lead to inaccurate results. The heat accelerates the reaction between the reducing sugar and Benedict's reagent.
-
Observe the Color Change: After heating, observe the color change in the solution. The color change indicates the presence and concentration of reducing sugars:
- Blue: No reducing sugar present. The solution remains the original blue color of Benedict's reagent.
- Green: A very small amount of reducing sugar is present.
- Yellow: A moderate amount of reducing sugar is present.
- Orange: A larger amount of reducing sugar is present.
- Brick-red/Brown: A very high concentration of reducing sugar is present.
Interpreting the Results: A Guide to Color Changes
The color change observed after heating provides qualitative information about the concentration of reducing sugars. While a precise quantitative measurement isn't possible with this test alone, the intensity of the color provides a relative indication of the amount of reducing sugar present.
Understanding the Color Spectrum: The color change is a result of the varying amounts of copper(I) oxide (Cu₂O) formed during the reduction reaction. A small amount of reducing sugar will produce only a small amount of Cu₂O, resulting in a green color. A large amount of reducing sugar will produce a significant amount of Cu₂O, leading to a brick-red or brown color.
Importance of Controls: It's crucial to include a positive control (a known reducing sugar solution) and a negative control (distilled water) in your experiment. This allows you to verify the effectiveness of the reagent and to confirm the interpretation of your results. A positive control should produce a brick-red precipitate, while a negative control should remain blue.
Limitations of Benedict's Test
While Benedict's test is widely used and reliable for detecting reducing sugars, it does have certain limitations:
- Qualitative, Not Quantitative: Benedict's test provides qualitative information about the presence of reducing sugars but doesn't offer precise quantitative data. More sophisticated methods are needed for accurate quantification.
- Interference from Other Substances: Some substances can interfere with the test, giving false positive or false negative results. For instance, certain compounds can reduce copper(II) ions, even in the absence of reducing sugars.
- Sensitivity: The test's sensitivity might be low for detecting very small amounts of reducing sugars. More sensitive methods are available for detecting trace amounts.
- Not Specific to a Single Sugar: The test detects various reducing sugars indiscriminately; it cannot distinguish between different types of reducing sugars.
Applications of Benedict's Test
Benedict's test has various applications in different fields:
- Clinical Diagnosis: It's used in diagnosing conditions like diabetes mellitus, where excessive glucose in urine can be detected using this test.
- Food Science: The test helps determine the sugar content in various food products.
- Biochemistry: Used in research laboratories for qualitative detection of reducing sugars in various biological samples.
- Educational Setting: Widely used in schools and colleges as a simple and demonstrative experiment to teach about reducing sugars and oxidation-reduction reactions.
Comparing Benedict's Test with Other Sugar Tests
Several other tests can detect the presence of sugars. Let's compare Benedict's test with some of them:
1. Fehling's Test: Similar to Benedict's test, Fehling's test also uses copper(II) ions to detect reducing sugars. However, Fehling's solution is less stable than Benedict's reagent, requiring preparation immediately before use. Fehling's test is also susceptible to interference from substances other than reducing sugars.
2. Barfoed's Test: This test is specific for monosaccharides. It uses copper(II) acetate in an acidic environment. Only monosaccharides will react quickly enough to produce a positive result.
3. Iodine Test: The iodine test is used to detect starch, a polysaccharide, not reducing sugars. It's based on the formation of a blue-black complex when iodine interacts with starch.
Conclusion: Benedict's Reagent – A Valuable Tool in Sugar Detection
Benedict's reagent test is a valuable tool for detecting the presence of reducing sugars. Its simplicity, cost-effectiveness, and relative ease of performance make it a widely used method in various applications. While it has limitations concerning quantitative measurements and specificity, its qualitative detection of reducing sugars remains highly relevant in numerous settings. Understanding the principles, procedure, and interpretation of this test is fundamental for anyone working with carbohydrates or sugar analysis. Coupled with other tests and techniques, it contributes significantly to a comprehensive understanding of sugar chemistry and biology.
Latest Posts
Latest Posts
-
Barium Chloride Reacts With Sodium Sulphate
Mar 21, 2025
-
What Is Half Of 1 1 2 Tsp
Mar 21, 2025
-
Which Is Not A Cranial Bone
Mar 21, 2025
-
What Type Of Symmetry Do Echinoderms Have
Mar 21, 2025
-
90 Is What Percent Of 120
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Benedict's Reagent Tests For The Presence Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
